Page 58 - Bio Engineering Approaches to Cancer Diagnosis and Treatment
P. 58
56 CHAPTER 3 Immune assay assisted cancer diagnostic
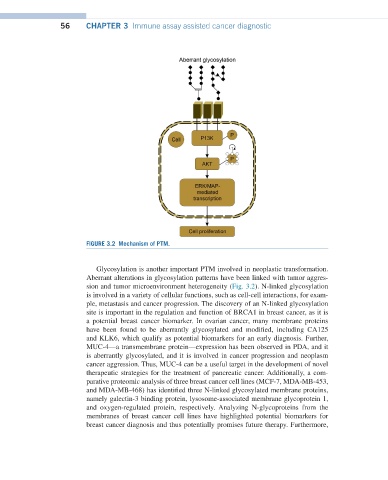
FIGURE 3.2 Mechanism of PTM.
Glycosylation is another important PTM involved in neoplastic transformation.
Aberrant alterations in glycosylation patterns have been linked with tumor aggres-
sion and tumor microenvironment heterogeneity (Fig. 3.2). N-linked glycosylation
is involved in a variety of cellular functions, such as cell-cell interactions, for exam-
ple, metastasis and cancer progression. The discovery of an N-linked glycosylation
site is important in the regulation and function of BRCA1 in breast cancer, as it is
a potential breast cancer biomarker. In ovarian cancer, many membrane proteins
have been found to be aberrantly glycosylated and modified, including CA125
and KLK6, which qualify as potential biomarkers for an early diagnosis. Further,
MUC-4—a transmembrane protein—expression has been observed in PDA, and it
is aberrantly glycosylated, and it is involved in cancer progression and neoplasm
cancer aggression. Thus, MUC-4 can be a useful target in the development of novel
therapeutic strategies for the treatment of pancreatic cancer. Additionally, a com-
parative proteomic analysis of three breast cancer cell lines (MCF-7, MDA-MB-453,
and MDA-MB-468) has identified three N-linked glycosylated membrane proteins,
namely galectin-3 binding protein, lysosome-associated membrane glycoprotein 1,
and oxygen-regulated protein, respectively. Analyzing N-glycoproteins from the
membranes of breast cancer cell lines have highlighted potential biomarkers for
breast cancer diagnosis and thus potentially promises future therapy. Furthermore,