Page 167 - Biofuels Refining and Performance
P. 167
150 Chapter Five
ignition quality, cold flow, oxidative stability, viscosity, and lubricity.
This chapter discusses the influence of the structure of fatty esters on
these properties. Not all of these properties have been included in
biodiesel standards, although all of them are essential to proper func-
tioning of the fuel in a diesel engine.
Generally, as the least expensive alcohol, methanol has been used to
produce biodiesel. Biodiesel, in most cases, can therefore be termed the
fatty acid methyl esters (FAME) of a vegetable oil or animal fat.
However, as mentioned above, both the fatty acid chain and the alcohol
functionality contribute to the overall properties of a fatty ester. It is
worthwhile to consider the properties imparted by other alcohols yield-
ing fatty acid alkyl esters (FAAE) that could be used for producing
biodiesel. Therefore, both structural moieties will be discussed in this
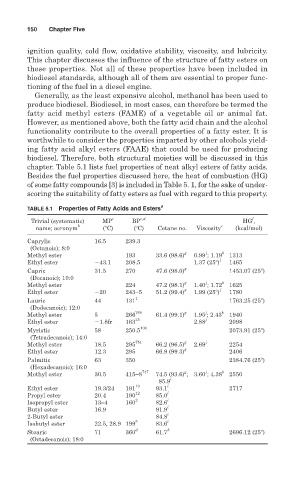
chapter. Table 5.1 lists fuel properties of neat alkyl esters of fatty acids.
Besides the fuel properties discussed here, the heat of combustion (HG)
of some fatty compounds [3] is included in Table 5. 1, for the sake of under-
scoring the suitability of fatty esters as fuel with regard to this property.
TABLE 5.1 Properties of Fatty Acids and Esters a
Trivial (systematic) MP c BP c,d HG , f
name; acronym b ( C) ( C) Cetane no. Viscosity e (kcal/mol)
Caprylic 16.5 239.3
(Octanoic); 8:0
j
Methyl ester 193 33.6 (98.6) g 0.99 ; 1.19 k 1313
Ethyl ester 43.1 208.5 1.37 (25 ) j 1465
Capric 31.5 270 47.6 (98.0) g 1453.07 (25 )
(Decanoic); 10:0
j
Methyl ester 224 47.2 (98.1) g 1.40 ; 1.72 k 1625
Ethyl ester 20 243–5 51.2 (99.4) g 1.99 (25 ) j 1780
Lauric 44 131 1 1763.25 (25 )
(Dodecanoic); 12:0
j
Methyl ester 5 266 766 61.4 (99.1) g 1.95 ; 2.43 k 1940
Ethyl ester 1.8fr 163 25 2.88 j 2098
Myristic 58 250.5 100 2073.91 (25 )
(Tetradecanoic); 14:0
Methyl ester 18.5 295 751 66.2 (96.5) g 2.69 j 2254
Ethyl ester 12.3 295 66.9 (99.3) g 2406
Palmitic 63 350 2384.76 (25 )
(Hexadecanoic); 16:0
j
g
Methyl ester 30.5 415–8 747 74.5 (93.6) ; 3.60 ; 4.38 k 2550
85.9 i
Ethyl ester 19.3/24 191 10 93.1 i 2717
Propyl ester 20.4 190 12 85.0 i
Isopropyl ester 13–4 160 2 82.6 i
Butyl ester 16.9 91.9 i
2-Butyl ester 84.8 i
Isobutyl ester 22.5, 28.9 199 5 83.6 i
Stearic 71 360 d 61.7 h 2696.12 (25 )
(Octadecanoic); 18:0