Page 186 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 186
Chapter | 5 Pyrolysis 163
Reaction II
Decarboxylation
Dehydration
carbonization
Reaction I
Active
Cellulose
cellulose
Reaction IV
Reaction III
Depolymerization Scission Secondary cracking
condensable gases Char, tar, non
condensable gases
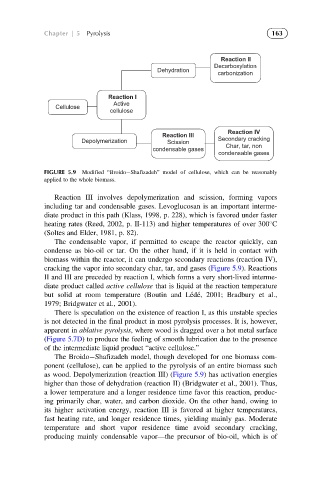
FIGURE 5.9 Modified “Broido Shafizadeh” model of cellulose, which can be reasonably
applied to the whole biomass.
Reaction III involves depolymerization and scission, forming vapors
including tar and condensable gases. Levoglucosan is an important interme-
diate product in this path (Klass, 1998, p. 228), which is favored under faster
heating rates (Reed, 2002, p. II-113) and higher temperatures of over 300 C
(Soltes and Elder, 1981, p. 82).
The condensable vapor, if permitted to escape the reactor quickly, can
condense as bio-oil or tar. On the other hand, if it is held in contact with
biomass within the reactor, it can undergo secondary reactions (reaction IV),
cracking the vapor into secondary char, tar, and gases (Figure 5.9). Reactions
II and III are preceded by reaction I, which forms a very short-lived interme-
diate product called active cellulose that is liquid at the reaction temperature
but solid at room temperature (Boutin and Le ´de ´, 2001; Bradbury et al.,
1979; Bridgwater et al., 2001).
There is speculation on the existence of reaction I, as this unstable species
is not detected in the final product in most pyrolysis processes. It is, however,
apparent in ablative pyrolysis, where wood is dragged over a hot metal surface
(Figure 5.7D) to produce the feeling of smooth lubrication due to the presence
of the intermediate liquid product “active cellulose.”
The Broido Shafizadeh model, though developed for one biomass com-
ponent (cellulose), can be applied to the pyrolysis of an entire biomass such
as wood. Depolymerization (reaction III) (Figure 5.9) has activation energies
higher than those of dehydration (reaction II) (Bridgwater et al., 2001). Thus,
a lower temperature and a longer residence time favor this reaction, produc-
ing primarily char, water, and carbon dioxide. On the other hand, owing to
its higher activation energy, reaction III is favored at higher temperatures,
fast heating rate, and longer residence times, yielding mainly gas. Moderate
temperature and short vapor residence time avoid secondary cracking,
producing mainly condensable vapor—the precursor of bio-oil, which is of