Page 197 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 197
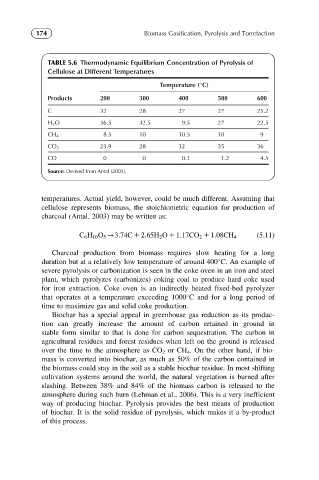
174 Biomass Gasification, Pyrolysis and Torrefaction
TABLE 5.6 Thermodynamic Equilibrium Concentration of Pyrolysis of
Cellulose at Different Temperatures
Temperature ( C)
Products 200 300 400 500 600
C 32 28 27 27 25.2
H 2 O 36.5 32.5 9.5 27 22.5
8.5 10 10.5 10 9
CH 4
CO 2 23.9 28 32 35 36
CO 0 0 0.1 1.2 4.5
Source: Derived from Antal (2003).
temperatures. Actual yield, however, could be much different. Assuming that
cellulose represents biomass, the stoichiometric equation for production of
charcoal (Antal, 2003) may be written as:
C 6 H 10 O 5 -3:74C 1 2:65H 2 O 1 1:17CO 2 1 1:08CH 4 (5.11)
Charcoal production from biomass requires slow heating for a long
duration but at a relatively low temperature of around 400 C. An example of
severe pyrolysis or carbonization is seen in the coke oven in an iron and steel
plant, which pyrolyzes (carbonizes) coking coal to produce hard coke used
for iron extraction. Coke oven is an indirectly heated fixed-bed pyrolyzer
that operates at a temperature exceeding 1000 C and for a long period of
time to maximize gas and solid coke production.
Biochar has a special appeal in greenhouse gas reduction as its produc-
tion can greatly increase the amount of carbon retained in ground in
stable form similar to that is done for carbon sequestration. The carbon in
agricultural residues and forest residues when left on the ground is released
over the time to the atmosphere as CO 2 or CH 4 . On the other hand, if bio-
mass is converted into biochar, as much as 50% of the carbon contained in
the biomass could stay in the soil as a stable biochar residue. In most shifting
cultivation systems around the world, the natural vegetation is burned after
slashing. Between 38% and 84% of the biomass carbon is released to the
atmosphere during such burn (Lehman et al., 2006). This is a very inefficient
way of producing biochar. Pyrolysis provides the best means of production
of biochar. It is the solid residue of pyrolysis, which makes it a by-product
of this process.