Page 334 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 334
BIOPOLYMERS 311
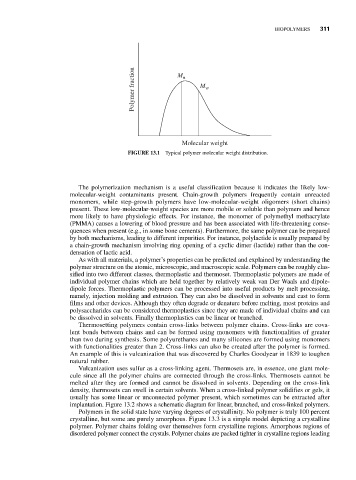
Polymer fraction M n M w
Molecular weight
FIGURE 13.1 Typical polymer molecular weight distribution.
The polymerization mechanism is a useful classification because it indicates the likely low-
molecular-weight contaminants present. Chain-growth polymers frequently contain unreacted
monomers, while step-growth polymers have low-molecular-weight oligomers (short chains)
present. These low-molecular-weight species are more mobile or soluble than polymers and hence
more likely to have physiologic effects. For instance, the monomer of polymethyl methacrylate
(PMMA) causes a lowering of blood pressure and has been associated with life-threatening conse-
quences when present (e.g., in some bone cements). Furthermore, the same polymer can be prepared
by both mechanisms, leading to different impurities. For instance, polylactide is usually prepared by
a chain-growth mechanism involving ring opening of a cyclic dimer (lactide) rather than the con-
densation of lactic acid.
As with all materials, a polymer’s properties can be predicted and explained by understanding the
polymer structure on the atomic, microscopic, and macroscopic scale. Polymers can be roughly clas-
sified into two different classes, thermoplastic and thermoset. Thermoplastic polymers are made of
individual polymer chains which are held together by relatively weak van Der Waals and dipole-
dipole forces. Thermoplastic polymers can be processed into useful products by melt processing,
namely, injection molding and extrusion. They can also be dissolved in solvents and cast to form
films and other devices. Although they often degrade or denature before melting, most proteins and
polysaccharides can be considered thermoplastics since they are made of individual chains and can
be dissolved in solvents. Finally thermoplastics can be linear or branched.
Thermosetting polymers contain cross-links between polymer chains. Cross-links are cova-
lent bonds between chains and can be formed using monomers with functionalities of greater
than two during synthesis. Some polyurethanes and many silicones are formed using monomers
with functionalities greater than 2. Cross-links can also be created after the polymer is formed.
An example of this is vulcanization that was discovered by Charles Goodyear in 1839 to toughen
natural rubber.
Vulcanization uses sulfur as a cross-linking agent. Thermosets are, in essence, one giant mole-
cule since all the polymer chains are connected through the cross-links. Thermosets cannot be
melted after they are formed and cannot be dissolved in solvents. Depending on the cross-link
density, thermosets can swell in certain solvents. When a cross-linked polymer solidifies or gels, it
usually has some linear or unconnected polymer present, which sometimes can be extracted after
implantation. Figure 13.2 shows a schematic diagram for linear, branched, and cross-linked polymers.
Polymers in the solid state have varying degrees of crystallinity. No polymer is truly 100 percent
crystalline, but some are purely amorphous. Figure 13.3 is a simple model depicting a crystalline
polymer. Polymer chains folding over themselves form crystalline regions. Amorphous regions of
disordered polymer connect the crystals. Polymer chains are packed tighter in crystalline regions leading