Page 306 - Biosystems Engineering
P. 306
Bioseparation Pr ocesses 283
A good bioseparation process should have the following properties:
1. Desired purity of the product
2. Stability of the product
3. Low cost
4. Reproducibility and scalability
5. Meet regulatory guidelines
Bioseparation processes have in common many familiar chemical
engineering unit operations (see Table 9.1). For example, aerobic fer-
mentations involve mixing three heterogeneous phases—microorgan-
ism, medium, and air. In the manufacture of antibiotics, mass transfer
(e.g., extraction, adsorption, and drying), heat transfer (such as evap-
oration, drying, and crystallization), and other mechanical operations
(e.g., cell rupture, settling thickening, filtration, and centrifuging) all
play a vital role. The investment on these operations is often claimed
to be about 4 times greater than that for the ferment or vessels and
their auxiliary equipment. Often, as much as 60 percent of the fixed
costs of fermentation are attributable to the recovery stage in organic
acid and amino acid production.
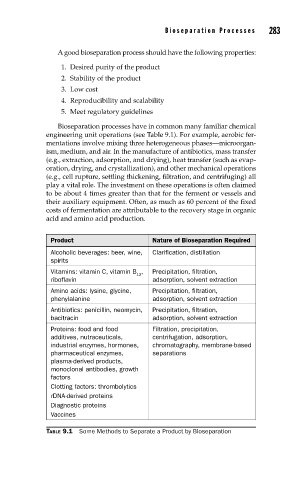
Product Nature of Bioseparation Required
Alcoholic beverages: beer, wine, Clarification, distillation
spirits
Vitamins: vitamin C, vitamin B , Precipitation, filtration,
12
riboflavin adsorption, solvent extraction
Amino acids: lysine, glycine, Precipitation, filtration,
phenylalanine adsorption, solvent extraction
Antibiotics: penicillin, neomycin, Precipitation, filtration,
bacitracin adsorption, solvent extraction
Proteins: food and food Filtration, precipitation,
additives, nutraceuticals, centrifugation, adsorption,
industrial enzymes, hormones, chromatography, membrane-based
pharmaceutical enzymes, separations
plasma-derived products,
monoclonal antibodies, growth
factors
Clotting factors: thrombolytics
rDNA-derived proteins
Diagnostic proteins
Vaccines
TABLE 9.1 Some Methods to Separate a Product by Bioseparation