Page 78 - Carbonate Sedimentology and Sequence Stratigraphy
P. 78
CHAPTER 4: CARBONATE FACIES MODELS 69
evolution; thus, complete separation of environmental and Bashkirian
evolutionary controls on the sediment record is impossible. Valdeteja Formation
Analysis of the functional morphology of organisms is one Spain
of the most successful attempts to extract purely environ-
mental messages from fossils (Dodd and Stanton, 1981, p.
222–261). 40
Fig. 4.17 show examples of information on water depth
and that can be extracted from fossils in carbonate rocks.
Note that the presentation remains at the level of high taxo- Section meter
nomic categories. These categories (for instance, gastropods,
echinoderms etc.) existed through most or all of the Phane- 30
rozoic. For more detail see the excellent exposes in Flügel
(2004) and Dodd and Stanton (1981, p. 17–115).
P
SILICICLASTICS AND EVAPORITES IN
CARBONATE FACIES 20
Siliciclastics and evaporites (e.g. gypsum, halite) may ap-
pear in carbonate deposits as in-bed admixtures, as beds
that alternate with carbonate beds and as formations that
interfinger laterally with carbonate formations. At the scale 10
of entire basins, phases of carbonate deposition may alter-
nate with phases of siliciclastic or evaporite deposition. The
details of these contacts and the effects of siliciclastics and
evaporites on carbonate systems differ and need to be ex-
amined separately. 0
Siliciclastics are transported into carbonate environments
from external sources. They may occur in any one of the -1 0 13 1 2 3
0
δ C ( /00 VPDB)
carbonate facies belts but the most occmon occurrence is at
the landward and seaward ends of the carbonate facies spec-
13
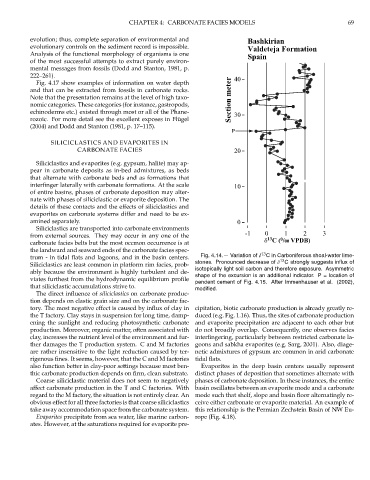
Fig. 4.14.— Variation of δ C in Carboniferous shoal-water lime-
trum - in tidal flats and lagoons, and in the basin centers. 13
Siliciclastics are least common in platform rim facies, prob- stones. Pronounced decrease of δ C strongly suggests influx of
ably because the environment is highly turbulent and de- isotopically light soil carbon and therefore exposure. Asymmetric
shape of the excursion is an additional indicator. P = location of
viates furthest from the hydrodynamic equilibrium profile pendent cement of Fig. 4.15. After Immenhauser et al. (2002),
that siliciclastic accumulations strive to. modified.
The direct influence of siliciclastics on carbonate produc-
tion depends on clastic grain size and on the carbonate fac-
tory. The most negative effect is caused by influx of clay in cipitation, biotic carbonate production is already greatly re-
the T factory. Clay stays in suspension for long time, damp- duced (e.g. Fig. 1.16). Thus, the sites of carbonate production
ening the sunlight and reducing photosynthetic carbonate and evaporite precipitation are adjacent to each other but
production. Moreover, organic matter, often associated with do not broadly overlap. Consequently, one observes facies
clay, increases the nutrient level of the environment and fur- interfingering, particularly between restricted carbonate la-
ther damages the T production system. C and M factories goons and sabkha evaporites (e.g. Sarg, 2001). Also, diage-
are rather insensitive to the light reduction caused by ter- netic admixtures of gypsum are common in arid carbonate
rigenous fines. It seems, however, that the C and M factories tidal flats.
also function better in clay-poor settings because most ben- Evaporites in the deep basin centers usually represent
thic carbonate production depends on firm, clean substrate. distinct phases of deposition that sometimes alternate with
Coarse siliciclastic material does not seem to negatively phases of carbonate deposition. In these instances, the entire
affect carbonate production in the T and C factories. With basin oscillates between an evaporite mode and a carbonate
regard to the M factory, the situation is not entirely clear. An mode such that shelf, slope and basin floor alternatingly re-
obvious effect for all three factories is that coarse siliciclastics ceive either carbonate or evaporite material. An example of
take away accommodation space from the carbonate system. this relationship is the Permian Zechstein Basin of NW Eu-
Evaporites precipitate from sea water, like marine carbon- rope (Fig. 4.18).
ates. However, at the saturations required for evaporite pre-