Page 345 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 345
308 Carraher’s Polymer Chemistry
cis-1,4-Polyisoprene (rubber) Isoprene
H C H 3 C H 2 C CH 3
3
R
R CH 2
alpha-trans-1,4-Polyisoprene; alpha-gutta-percha
R R R
CH 3 CH 3 R
R CH 2 H 3 C CH 2
H C R 1,2-Product 3,4-Product
3
beta-trans-1,4-Polyisoprene; beta-gutta-percha R R
R R R
CH 3 CH 3 CH 3 H C
3
H C R
cis-1,4-Product 3
trans-1,4-Product
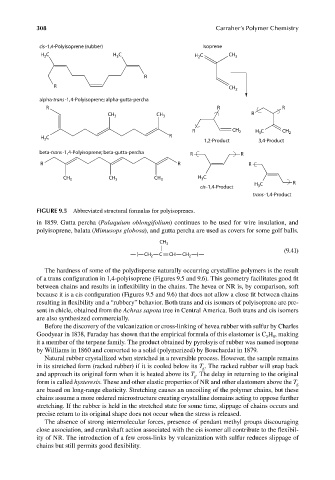
FIGURE 9.5 Abbreviated structural formulas for polyisoprenes.
in 1859. Gutta percha (Palaquium oblongifolium) continues to be used for wire insulation, and
polyisoprene, balata (Mimusops globosa), and gutta percha are used as covers for some golf balls.
CH 3
| (9.41)
2
| ( CH C CH CH 2 ) |
|
|
|
|
The hardness of some of the polydisperse naturally occurring crystalline polymers is the result
of a trans configuration in 1,4-polyisoprene (Figures 9.5 and 9.6). This geometry facilitates good fi t
between chains and results in inflexibility in the chains. The hevea or NR is, by comparison, soft
because it is a cis configuration (Figures 9.5 and 9.6) that does not allow a close fit between chains
resulting in flexibility and a “rubbery” behavior. Both trans and cis isomers of polyisoprene are pre-
sent in chicle, obtained from the Achras sapota tree in Central America. Both trans and cis isomers
are also synthesized commercially.
Before the discovery of the vulcanization or cross-linking of hevea rubber with sulfur by Charles
Goodyear in 1838, Faraday has shown that the empirical formula of this elastomer is C H , making
8
5
it a member of the terpene family. The product obtained by pyrolsyis of rubber was named isoprene
by Williams in 1860 and converted to a solid (polymerized) by Bouchardat in 1879.
Natural rubber crystallized when stretched in a reversible process. However, the sample remains
in its stretched form (racked rubber) if it is cooled below its T . The racked rubber will snap back
g
and approach its original form when it is heated above its T . The delay in returning to the original
g
form is called hysteresis. These and other elastic properties of NR and other elastomers above the T
g
are based on long-range elasticity. Stretching causes an uncoiling of the polymer chains, but these
chains assume a more ordered microstructure creating crystalline domains acting to oppose further
stretching. If the rubber is held in the stretched state for some time, slippage of chains occurs and
precise return to its original shape does not occur when the stress is released.
The absence of strong intermolecular forces, presence of pendant methyl groups discouraging
close association, and crankshaft action associated with the cis isomer all contribute to the fl exibil-
ity of NR. The introduction of a few cross-links by vulcanization with sulfur reduces slippage of
chains but still permits good fl exibility.
9/14/2010 3:40:52 PM
K10478.indb 308 9/14/2010 3:40:52 PM
K10478.indb 308