Page 570 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 570
Reactions on Polymers 533
CH 2
O
O
N CH 3
O
N
(16.21)
CH 2
H C – OH
3
O
N
N
O
H C
2
CH 2
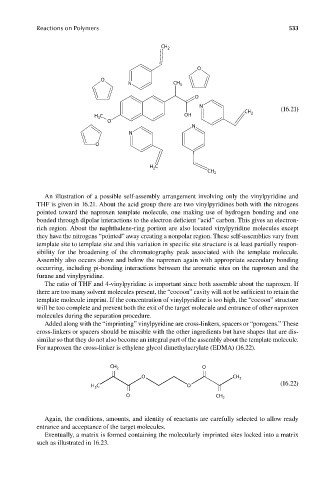
An illustration of a possible self-assembly arrangement involving only the vinylpyridine and
THF is given in 16.21. About the acid group there are two vinylpyridines both with the nitrogens
pointed toward the naproxen template molecule, one making use of hydrogen bonding and one
bonded through dipolar interactions to the electron deficient “acid” carbon. This gives an electron-
rich region. About the naphthalene-ring portion are also located vinylpyridine molecules except
they have the nitrogens “pointed” away creating a nonpolar region. These self-assemblies vary from
template site to template site and this variation in specific site structure is at least partially respon-
sibility for the broadening of the chromatography peak associated with the template molecule.
Assembly also occurs above and below the naproxen again with appropriate secondary bonding
occurring, including pi-bonding interactions between the aromatic sites on the naproxen and the
furane and vinylpyridine.
The ratio of THF and 4-vinylpyridine is important since both assemble about the naproxen. If
there are too many solvent molecules present, the “cocoon” cavity will not be sufficient to retain the
template molecule imprint. If the concentration of vinylpyridine is too high, the “cocoon” structure
will be too complete and prevent both the exit of the target molecule and entrance of other naproxen
molecules during the separation procedure.
Added along with the “imprinting” vinylpyridine are cross-linkers, spacers or “porogens.” These
cross-linkers or spacers should be miscible with the other ingredients but have shapes that are dis-
similar so that they do not also become an integral part of the assembly about the template molecule.
For naproxen the cross-linker is ethylene glycol dimethylacrylate (EDMA) (16.22).
CH O
– – 2 O – – CH 3
(16.22)
H C – – O – –
3
O CH
2
Again, the conditions, amounts, and identity of reactants are carefully selected to allow ready
entrance and acceptance of the target molecules.
Eventually, a matrix is formed containing the molecularly imprinted sites locked into a matrix
such as illustrated in 16.23.
9/14/2010 3:43:08 PM
K10478.indb 533 9/14/2010 3:43:08 PM
K10478.indb 533