Page 695 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 695
658 Carraher’s Polymer Chemistry
As our bioengineering revolution matures it should be possible to employ various biomasses as
resources that can be converted into classical monomers such as ethylene, propylene, styrene, tere-
phthalic acid, and so on from reactions caused by genetically designed microbes. In fact, it may be
possible for combinations of microbes to produce finished polymers that may have better tacticity
and chain orientation producing polymers with superior physical properties.
The latest major emphasis on a major green material is PLA discussed in the previous section.
The use of rayon and other material derived from cellulose has also been discussed.
There are many vegetable oils that are currently available on an industrial scale. These include
palm, soybean, cotton seed, castor, and rapeseed oils. While these have been employed in the pro-
duction of many commercial materials such as coatings, pharmaceuticals, plasticizers, and building
materials, the particular fatty acids are being used as polymeric materials. Three of the fi ve most
common fatty acid substituents, oleic acid, linoleic acid, and linolenic acid, are unsaturated with
these sites of unsaturation available for cross-linking. These are pictured in Figure 19.11. These fatty
acids have been directly polymerized through the double bond(s).
Castor oil produces ricinoleic acid on its hydrolysis. Ricinoleic acid has a single olefi nic site
as well as both an acid and alcohol group (Figure 19.11). Thus, it can be polymerized by reaction
through its double bond or by condensation, as shown by Carraher and workers, with a Lewis acid.
Polyesters have also been produced through formation of the lactone followed by ROP.
One area of activity involves the production of polyurethanes employing fatty acids as well as the oils
themselves. Often the oil is functionalized with hydroxyl groups through reaction at the unsaturated sites.
The reaction is analogous to that employed to produce a number of soft-hard block polyurethanes.
Carbon dioxide is readily available and renewable. It is employed in the production of PC but can
also be utilized in the production of various polymers through reaction with different heterocycles
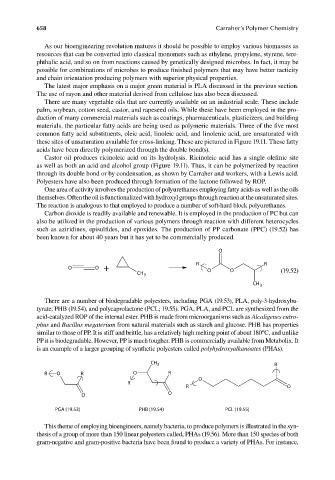
such as aziridines, episulfides, and epoxides. The production of PP carbonate (PPC) (19.52) has
been known for about 40 years but it has yet to be commercially produced.
O
R R
O O + O O
CH 3 (19.52)
CH 3
There are a number of biodegradable polyesters, including PGA (19.53), PLA, poly-3-hydroxybu-
tyrate; PHB (19.54), and polycaprolactone (PCL; 19.55). PGA, PLA, and PCL are synthesized from the
acid-catalyzed ROP of the internal ester. PHB is made from microorganisms such as Alcaligenes eutro-
phus and Bacillus megaterium from natural materials such as starch and glucose. PHB has properties
similar to those of PP. It is stiff and brittle, has a relatively high melting point of about 180ºC, and unlike
PP it is biodegradable. However, PP is much tougher. PHB is commercially available from Metabolix. It
is an example of a larger grouping of synthetic polyesters called polyhydroxyalkanoates (PHAs).
CH 3 R
R O R O R
O
R
R O
O O
PGA (19.53) PHB (19.54) PCL (19.55)
This theme of employing bioengineers, namely bacteria, to produce polymers is illustrated in the syn-
thesis of a group of more than 150 linear polyesters called, PHAs (19.56). More than 150 species of both
gram-negative and gram-positive bacteria have been found to produce a variety of PHAs. For instance,
9/14/2010 3:44:08 PM
K10478.indb 658 9/14/2010 3:44:08 PM
K10478.indb 658