Page 209 - Cascade biocatalysis
P. 209
8.3 Applications of DKR to Acyl Compounds 185
NH 2 NH 3 + NH + NH 3 +
O − O − O − OMe
H H H H H
N +
O O O O
pK a 34 29 22 21 O −
NH +
O −
H
+ O
NMe 3 +
NH + O − NH − pK 17
OMe H H O a
H
O
O O
pK a 14 27 23
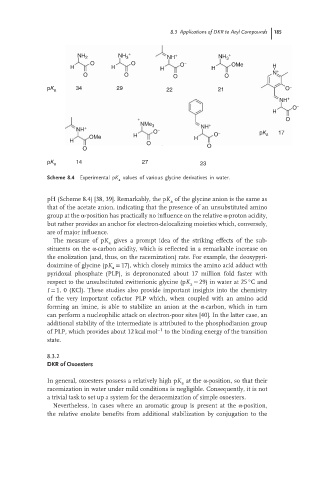
Scheme 8.4 Experimental pK values of various glycine derivatives in water.
a
pH (Scheme 8.4) [38, 39]. Remarkably, the pK of the glycine anion is the same as
a
that of the acetate anion, indicating that the presence of an unsubstituted amino
group at the α-position has practically no influence on the relative α-proton acidity,
but rather provides an anchor for electron-delocalizing moieties which, conversely,
are of major influence.
ThemeasureofpK gives a prompt idea of the striking effects of the sub-
a
stituents on the α-carbon acidity, which is reflected in a remarkable increase on
the enolization (and, thus, on the racemization) rate. For example, the deoxypyri-
doximine of glycine (pK = 17), which closely mimics the amino acid adduct with
a
pyridoxal phosphate (PLP), is deprononated about 17 million fold faster with
◦
respect to the unsubstituted zwitterionic glycine (pK = 29) in water at 25 Cand
a
I = 1, 0 (KCl). These studies also provide important insights into the chemistry
of the very important cofactor PLP which, when coupled with an amino acid
forming an imine, is able to stabilize an anion at the α-carbon, which in turn
can perform a nucleophilic attack on electron-poor sites [40]. In the latter case, an
additional stability of the intermediate is attributed to the phosphodianion group
of PLP, which provides about 12 kcal mol −1 to the binding energy of the transition
state.
8.3.2
DKR of Oxoesters
In general, oxoesters possess a relatively high pK at the α-position, so that their
a
racemization in water under mild conditions is negligible. Consequently, it is not
a trivial task to set up a system for the deracemization of simple oxoesters.
Nevertheless, in cases where an aromatic group is present at the α-position,
the relative enolate benefits from additional stabilization by conjugation to the