Page 275 - Cascade biocatalysis
P. 275
11.2 Natural Cascades 251
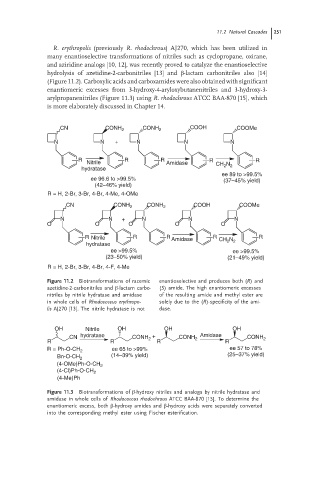
R. erythropolis (previously R. rhodochrous) AJ270, which has been utilized in
many enantioselective transformations of nitriles such as cyclopropane, oxirane,
and aziridine analogs [10, 12], was recently proved to catalyze the enantioselective
hydrolysis of azetidine-2-carbonitriles [13] and β-lactam carbonitriles also [14]
(Figure 11.2). Carboxylic acids and carboxamides were also obtained with significant
enantiomeric excesses from 3-hydroxy-4-aryloxybutanenitriles and 3-hydroxy-3-
arylpropanenitriles (Figure 11.3) using R. rhodochrous ATCC BAA-870 [15], which
is more elaborately discussed in Chapter 14.
CN CONH 2 CONH 2 COOH COOMe
N N + N N N
R R R R R
Nitrile Amidase CH N
hydratase 2 2
ee 89 to >99.5%
ee 96.6 to >99.5% (37–45% yield)
(42–46% yield)
R = H, 2-Br, 3-Br, 4-Br, 4-Me, 4-OMe
CN CONH 2 CONH 2 COOH COOMe
N N + N N N
O O O O O
R Nitrile R R Amidase R CH N 2 R
hydratase 2
ee >99.5% ee >99.5%
(23–50% yield) (21–49% yield)
R = H, 2-Br, 3-Br, 4-Br, 4-F, 4-Me
Figure 11.2 Biotransformations of racemic enantioselective and produces both (R)and
azetidine-2-carbonitriles and β-lactam carbo- (S) amide. The high enantiomeric excesses
nitriles by nitrile hydratase and amidase of the resulting amide and methyl ester are
in whole cells of Rhodococcus erythropo- solely due to the (R)-specificity of the ami-
lis AJ270 [13]. The nitrile hydratase is not dase.
OH Nitrile OH OH OH
CN hydratase CONH + CONH 2 Amidase CONH 2
2
R R R R
ee 65 to >99% ee 57 to 78%
R = Ph-O-CH 2
Bn-O-CH 2 (14–39% yield) (25–37% yield)
(4-OMe)Ph-O-CH 2
(4-Cl)Ph-O-CH 2
(4-Me)Ph
Figure 11.3 Biotransformations of β-hydroxy nitriles and analogs by nitrile hydratase and
amidase in whole cells of Rhodococcus rhodochrous ATCC BAA-870 [15]. To determine the
enantiomeric excess, both β-hydroxy amides and β-hydroxy acids were separately converted
into the corresponding methyl ester using Fischer esterification.