Page 35 - Chalcogenide Glasses for Infrared Optics
P. 35
14 Cha pte r O n e
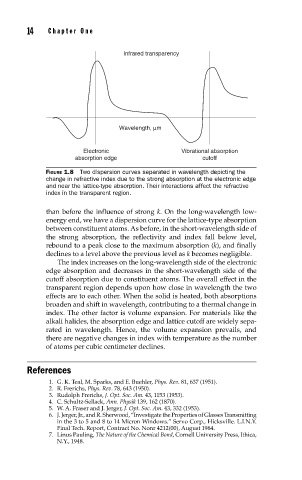
Infrared transparency
Wavelength, µm
Electronic Vibrational absorption
absorption edge cutoff
FIGURE 1.8 Two dispersion curves separated in wavelength depicting the
change in refractive index due to the strong absorption at the electronic edge
and near the lattice-type absorption. Their interactions affect the refractive
index in the transparent region.
than before the influence of strong k. On the long-wavelength low-
energy end, we have a dispersion curve for the lattice-type absorption
between constituent atoms. As before, in the short-wavelength side of
the strong absorption, the reflectivity and index fall below level,
rebound to a peak close to the maximum absorption (k), and finally
declines to a level above the previous level as k becomes negligible.
The index increases on the long-wavelength side of the electronic
edge absorption and decreases in the short-wavelength side of the
cutoff absorption due to constituent atoms. The overall effect in the
transparent region depends upon how close in wavelength the two
effects are to each other. When the solid is heated, both absorptions
broaden and shift in wavelength, contributing to a thermal change in
index. The other factor is volume expansion. For materials like the
alkali halides, the absorption edge and lattice cutoff are widely sepa-
rated in wavelength. Hence, the volume expansion prevails, and
there are negative changes in index with temperature as the number
of atoms per cubic centimeter declines.
References
1. G. K. Teal, M. Sparks, and E. Buehler, Phys. Rev. 81, 637 (1951).
2. R. Frerichs, Phys. Rev. 78, 643 (1950).
3. Rudolph Frerichs, J. Opt. Soc. Am. 43, 1153 (1953).
4. C. Schultz-Sellack, Ann. Physik 139, 162 (1870).
5. W. A. Fraser and J. Jerger, J. Opt. Soc. Am. 43, 332 (1953).
6. J. Jerger, Jr., and R. Sherwood, “Investigate the Properties of Glasses Transmitting
in the 3 to 5 and 8 to 14 Micron Windows.” Servo Corp., Hicksville. L.I.N.Y.
Final Tech. Report, Contract No. Nonr 4212(00), August 1964.
7. Linus Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithica,
N.Y., 1948.