Page 104 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 104
82 INTRODUCTION AND FORMS OF CORROSION
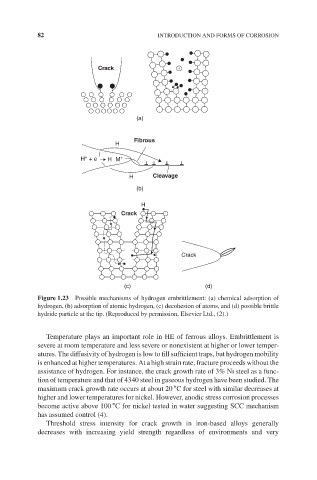
Crack
(a)
Fibrous
H
+
H + e H M +
H Cleavage
(b)
H
Crack
Crack
(c) (d)
Figure 1.23 Possible mechanisms of hydrogen embrittlement: (a) chemical adsorption of
hydrogen, (b) adsorption of atomic hydrogen, (c) decohesion of atoms, and (d) possible brittle
hydride particle at the tip. (Reproduced by permission, Elsevier Ltd., (2).)
Temperature plays an important role in HE of ferrous alloys. Embrittlement is
severe at room temperature and less severe or nonexistent at higher or lower temper-
atures. The diffusivity of hydrogen is low to fill sufficient traps, but hydrogen mobility
is enhanced at higher temperatures. At a high strain rate, fracture proceeds without the
assistance of hydrogen. For instance, the crack growth rate of 3% Ni steel as a func-
tion of temperature and that of 4340 steel in gaseous hydrogen have been studied. The
∘
maximum crack growth rate occurs at about 20 C for steel with similar decreases at
higher and lower temperatures for nickel. However, anodic stress corrosion processes
∘
become active above 100 C for nickel tested in water suggesting SCC mechanism
has assumed control (4).
Threshold stress intensity for crack growth in iron-based alloys generally
decreases with increasing yield strength regardless of environments and very