Page 268 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 268
246 CORROSION CONTROL AND PREVENTION
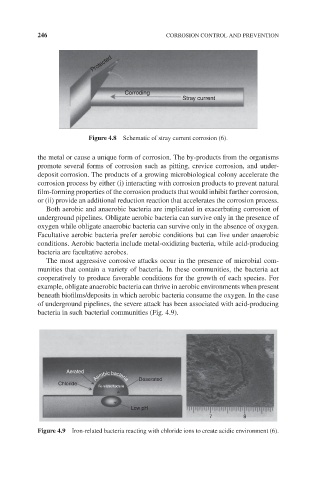
Protected
Corroding
Stray current
Figure 4.8 Schematic of stray current corrosion (6).
the metal or cause a unique form of corrosion. The by-products from the organisms
promote several forms of corrosion such as pitting, crevice corrosion, and under-
deposit corrosion. The products of a growing microbiological colony accelerate the
corrosion process by either (i) interacting with corrosion products to prevent natural
film-forming properties of the corrosion products that would inhibit further corrosion,
or (ii) provide an additional reduction reaction that accelerates the corrosion process.
Both aerobic and anaerobic bacteria are implicated in exacerbating corrosion of
underground pipelines. Obligate aerobic bacteria can survive only in the presence of
oxygen while obligate anaerobic bacteria can survive only in the absence of oxygen.
Facultative aerobic bacteria prefer aerobic conditions but can live under anaerobic
conditions. Aerobic bacteria include metal-oxidizing bacteria, while acid-producing
bacteria are facultative aerobes.
The most aggressive corrosive attacks occur in the presence of microbial com-
munities that contain a variety of bacteria. In these communities, the bacteria act
cooperatively to produce favorable conditions for the growth of each species. For
example, obligate anaerobic bacteria can thrive in aerobic environments when present
beneath biofilms/deposits in which aerobic bacteria consume the oxygen. In the case
of underground pipelines, the severe attack has been associated with acid-producing
bacteria in such bacterial communities (Fig. 4.9).
Aerated
Deaerated
Aerobic bacteria
Chloride
Fe-related bacteria
Low pH
7 8
Figure 4.9 Iron-related bacteria reacting with chloride ions to create acidic environment (6).