Page 48 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 48
26 INTRODUCTION AND FORMS OF CORROSION
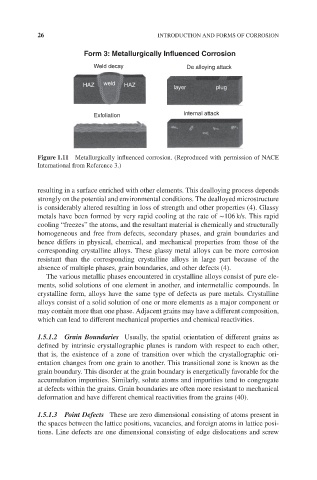
Form 3: Metallurgically Influenced Corrosion
Weld decay De alloying attack
HAZ weld HAZ
layer plug
Exfoliation Internal attack
Figure 1.11 Metallurgically influenced corrosion. (Reproduced with permission of NACE
International from Reference 3.)
resulting in a surface enriched with other elements. This dealloying process depends
strongly on the potential and environmental conditions. The dealloyed microstructure
is considerably altered resulting in loss of strength and other properties (4). Glassy
metals have been formed by very rapid cooling at the rate of ∼106 k/s. This rapid
cooling “freezes” the atoms, and the resultant material is chemically and structurally
homogeneous and free from defects, secondary phases, and grain boundaries and
hence differs in physical, chemical, and mechanical properties from those of the
corresponding crystalline alloys. These glassy metal alloys can be more corrosion
resistant than the corresponding crystalline alloys in large part because of the
absence of multiple phases, grain boundaries, and other defects (4).
The various metallic phases encountered in crystalline alloys consist of pure ele-
ments, solid solutions of one element in another, and intermetallic compounds. In
crystalline form, alloys have the same type of defects as pure metals. Crystalline
alloys consist of a solid solution of one or more elements as a major component or
may contain more than one phase. Adjacent grains may have a different composition,
which can lead to different mechanical properties and chemical reactivities.
1.5.1.2 Grain Boundaries Usually, the spatial orientation of different grains as
defined by intrinsic crystallographic planes is random with respect to each other,
that is, the existence of a zone of transition over which the crystallographic ori-
entation changes from one grain to another. This transitional zone is known as the
grain boundary. This disorder at the grain boundary is energetically favorable for the
accumulation impurities. Similarly, solute atoms and impurities tend to congregate
at defects within the grains. Grain boundaries are often more resistant to mechanical
deformation and have different chemical reactivities from the grains (40).
1.5.1.3 Point Defects These are zero dimensional consisting of atoms present in
the spaces between the lattice positions, vacancies, and foreign atoms in lattice posi-
tions. Line defects are one dimensional consisting of edge dislocations and screw