Page 95 - Chemical engineering design
P. 95
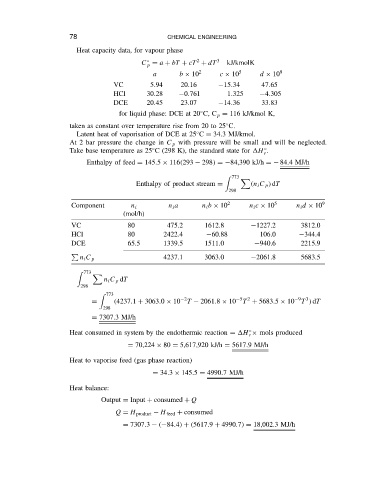
78
CHEMICAL ENGINEERING
Heat capacity data, for vapour phase
Ž
2
C D a C bT C cT C dT 3 kJ/kmolK
p
a b ð 10 2 c ð 10 5 d ð 10 9
VC 5.94 20.16 15.34 47.65
HCl 30.28 0.761 1.325 4.305
DCE 20.45 23.07 14.36 33.83
Ž
for liquid phase: DCE at 20 C, C p D 116 kJ/kmol K,
Ž
taken as constant over temperature rise from 20 to 25 C.
Ž
Latent heat of vaporisation of DCE at 25 C D 34.3 MJ/kmol.
At 2 bar pressure the change in C p with pressure will be small and will be neglected.
Ž
Ž
Take base temperature as 25 C (298 K), the standard state for H .
r
Enthalpy of feed D 145.5 ð 116 293 298 D 84,390 kJ/h D 84.4MJ/h
773
Enthalpy of product stream D n i C p dT
298
Component n i n i a n i b ð 10 2 n i c ð 10 5 n i d ð 10 9
(mol/h)
VC 80 475.2 1612.8 1227.2 3812.0
HCl 80 2422.4 60.88 106.0 344.4
DCE 65.5 1339.5 1511.0 940.6 2215.9
n i C p 4237.1 3063.0 2061.8 5683.5
773
n i C p dT
298
773
2
5
2
9
3
D 4237.1 C 3063.0 ð 10 T 2061.8 ð 10 T C 5683.5 ð 10 T dT
298
D 7307.3MJ/h
Ž
Heat consumed in system by the endothermic reaction D H ð mols produced
r
D 70,224 ð 80 D 5,617,920 kJ/h D 5617.9MJ/h
Heat to vaporise feed (gas phase reaction)
D 34.3 ð 145.5 D 4990.7MJ/h
Heat balance:
Output D Input C consumed C Q
Q D H product H feed C consumed
D 7307.3 84.4 C 5617.9 C 4990.7 D 18,002.3 MJ/h