Page 17 - Chemical equilibria Volume 4
P. 17
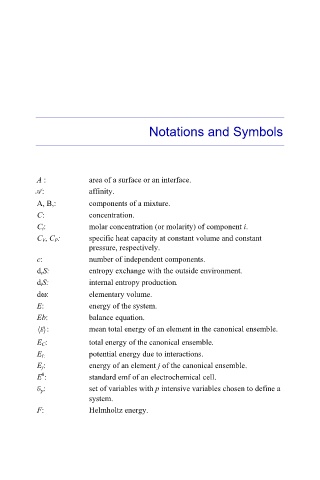
Notations and Symbols
A : area of a surface or an interface.
A: affinity.
A, B,: components of a mixture.
C: concentration.
C i: molar concentration (or molarity) of component i.
C V, C P: specific heat capacity at constant volume and constant
pressure, respectively.
c: number of independent components.
d eS: entropy exchange with the outside environment.
d iS: internal entropy production.
dω: elementary volume.
E: energy of the system.
Eb: balance equation.
E : mean total energy of an element in the canonical ensemble.
E C: total energy of the canonical ensemble.
E I: potential energy due to interactions.
E j: energy of an element j of the canonical ensemble.
0
E : standard emf of an electrochemical cell.
E p: set of variables with p intensive variables chosen to define a
system.
F: Helmholtz energy.