Page 26 - Chemical equilibria Volume 4
P. 26
2 Chemical Equilibria
This formulation shows the algebraic stoichiometric numbers ν , which
i
are counted as positive for the end components and as negative for the initial
components. The sum is extended to all of the initial and final components.
Physical transformations are characterized by the unit value of all the
stoichiometric numbers.
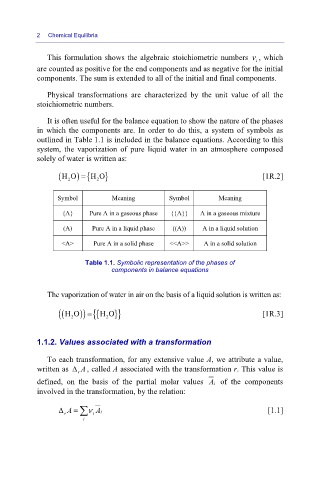
It is often useful for the balance equation to show the nature of the phases
in which the components are. In order to do this, a system of symbols as
outlined in Table 1.1 is included in the balance equations. According to this
system, the vaporization of pure liquid water in an atmosphere composed
solely of water is written as:
(HO ) {H O= 2 } [1R.2]
2
Symbol Meaning Symbol Meaning
{A} Pure A in a gaseous phase {{A}} A in a gaseous mixture
(A) Pure A in a liquid phase ((A)) A in a liquid solution
<A> Pure A in a solid phase <<A>> A in a solid solution
Table 1.1. Symbolic representation of the phases of
components in balance equations
The vaporization of water in air on the basis of a liquid solution is written as:
( ( HO )) { { H O= 2 }} [1R.3]
2
1.1.2. Values associated with a transformation
To each transformation, for any extensive value A, we attribute a value,
written as Δ A , called A associated with the transformation r. This value is
r
defined, on the basis of the partial molar values A of the components
i
involved in the transformation, by the relation:
Δ A = ∑ ν i i A [1.1]
r
i