Page 54 - Chemical equilibria Volume 4
P. 54
30 Chemical Equilibria
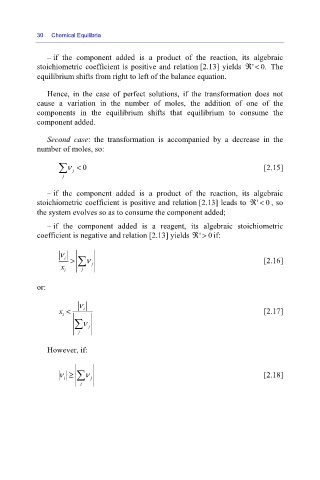
– if the component added is a product of the reaction, its algebraic
stoichiometric coefficient is positive and relation [2.13] yields ℜ<
'0. The
equilibrium shifts from right to left of the balance equation.
Hence, in the case of perfect solutions, if the transformation does not
cause a variation in the number of moles, the addition of one of the
components in the equilibrium shifts that equilibrium to consume the
component added.
Second case: the transformation is accompanied by a decrease in the
number of moles, so:
∑ ν < 0 [2.15]
j
j
– if the component added is a product of the reaction, its algebraic
stoichiometric coefficient is positive and relation [2.13] leads to ℜ< , so
'0
the system evolves so as to consume the component added;
– if the component added is a reagent, its algebraic stoichiometric
coefficient is negative and relation [2.13] yields ℜ> if:
'0
ν i > ∑ ν [2.16]
x i j j
or:
ν
x < i [2.17]
i
∑ ν j
j
However, if:
i ∑
ν ≥ ν j [2.18]
j