Page 149 - Chemical process engineering design and economics
P. 149
132 Chapter 3
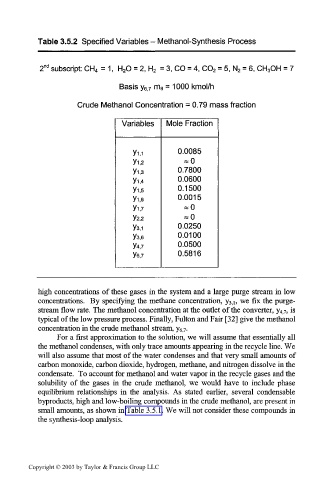
Table 3.5.2 Specified Variables - Methanol-Synthesis Process
na
2 subscript: CH 4 = 1, H 2O = 2, H 2 = 3, CO = 4, CO 2 = 5, N 2 = 6, CH 3OH = 7
Basis y 6]7 m 6 = 1000 kmol/h
Crude Methanol Concentration = 0.79 mass fraction
Variables Mole Fraction
0.0085
Y1.2 *0
Yi,3 0.7800
0.0600
Y1,4
Y1,5 0.1500
Y1,6 0.0015
Vi,7
Y2,2
0.0250
Y3.1
Y3.6 0.0100
Y4,7 0.0500
Ye,7 0.5816
high concentrations of these gases in the system and a large purge stream in low
concentrations. By specifying the methane concentration, y 3;], we fix the purge-
stream flow rate. The methanol concentration at the outlet of the converter, y 4j7, is
typical of the low pressure process. Finally, Fulton and Fair [32] give the methanol
concentration in the crude methanol stream, y 6, 7.
For a first approximation to the solution, we will assume that essentially all
the methanol condenses, with only trace amounts appearing in the recycle line. We
will also assume that most of the water condenses and that very small amounts of
carbon monoxide, carbon dioxide, hydrogen, methane, and nitrogen dissolve in the
condensate. To account for methanol and water vapor in the recycle gases and the
solubility of the gases in the crude methanol, we would have to include phase
equilibrium relationships in the analysis. As stated earlier, several condensable
byproducts, high and low-boiling compounds in the crude methanol, are present in
small amounts, as shown in Table 3.5.1. We will not consider these compounds in
the synthesis-loop analysis.
Copyright © 2003 by Taylor & Francis Group LLC