Page 230 - Chemical process engineering design and economics
P. 230
212 Chapter 5
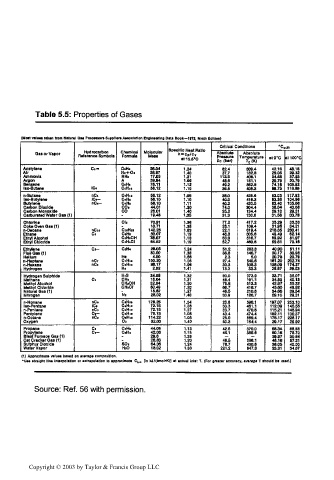
Table 5.5: Properties of Gases
(Mo« values taken from Natural QM Processors Suppliers Association Engineering Data Book—1972, Ninth Edition)
Critical Conditions *Cp.m
Absolute
Gas or Vapor Reference Symbole Chemlcal Molecular Specific Heat Ratio Pressure Te Absolute
k-cp/c»
Formula
Meat
ans.S'C PC (bar) T(Kr at 0-0 it 100'C
Acetylene Ci- C,H, 26.04 1.24 62.4 309.4 42.16 48.18
Air Nj-t-Oi 28.97 1.40 37.7 132.8 29.05 29.32
Ammonia NHi 17.03 1.31 112.6 406.1 34.65 37.93
Argon A 39.94 1.66 48.6 151.1 20.79 20.79
Benzene C.H. 78.11 1.12 49.2 562.8 74.18 103.62
Iso-Butane IC. C4H,« 58.12 1.10 36.6 408.3 89.75 116.89
n-Butane nC. CUHn 68.12 1.09 38.0 425.6 93.03 117.92
lao-Butylme 1C.— C4H. 68.10 1.10 40.0 418.3 83.36 104.98
Butylena nC4- C4Hi 68.10 1.11 40.2 420.0 83.40 105.08
Carbon Dioxide COi 44.01 1.30 74.0 304.4 36.04 40.06
Carbon Monoxlo> CO 28.01 1.40 35.2 134.4 29.10 29.31
Carbureted Water Gas (1) 19.48 1.35 31.3 130.6 31.58 33.78
Chlorine Cli 70.11 1.36 77.2 417.2 35.29 35.53
Coke Oven Qaefl) 10.71 1.35 26.1 109.4 31-95 34.21
n-Daoane nCi« Ci.Hn 142.28 1,03 22.1 619.4 21 855 280.41
Ethane 0, C.H. 30X17 1.19 48.8 305.6 49.49 62.14
Ethyl Alcohol CiHiOH tern 1.13 S3.9 516.7 69.92 81.97
Ethyl Chloride C.K.CI 64.52 1.19 52.7 460.6 69.61 70.16
Ethylene Ci- c.m 28.05 1.24 51.2 283.3 40.90 51.11
Flue Oat (1) 30.00 1.38 38.8 146.7 30.17 30.88
Helium He 4.00 1.68 2.3 5.0 20.79 20.79
n-Heptane nCj C7H» 100.20 1.05 27.4 540.6 161.20 202.74
n-Hexane r>C4 C.HI. 88.17 1.06 30.3 506.3 138.09 174.27
Hydrogen H, 2.02 1.41 13.0 33.3 28.87 29.03
Hydrogen Sulphide H.S 34.08 1.32 90.0 373.9 33.71 35.07
Methane Ci CH. 16.04 1.31 46.4 191.1 34.50 40.13
Methyl Alcohol CHjOH 32.04 1.20 79.8 513.3 42.67 55.32
Methyl Chloride CH.CI 50.49 1.20 86.7 416.7 45.60 49.82
Natural Qa»(1) 18.82 1.27 46.5 210.6 34.66 39.54
Nitrogen Ni 28.02 1.40 33.9 126.7 29.10 29.31
n-Nonane nC. C.H.. 128.25 1.04 23.8 596.1 197 .07 253.10
lao-Pentane ICi C.H,i 72.15 1.06 33.3 461.1 112.09 145.56
n-Pentane nCt C.H,, 72.15 1.07 33.7 470.6 115.21 145.94
Pentylene C.- C.H,. 70.13 1.08 40.4 474.4 102.11 130.37
n-Octane nC« C.H,. 114.22 1.05 25.0 569.4 176.17 226.17
Oxygen Oi 32.00 1.40 50.3 154.4 29.17 294)2
Propane Ci CiHi 44.09 1.13 42.5 370.0 68.34 86.68
Propylene Ci- C>H. 42.08 1.15 46.1 365.6 60.16 75.70
Blast Furnace Gaa (1) 29.6 1.39 29.97 30.64
Cat Cracker Gas(1) 28.83 1.20 48.5 288.1 46.16 57.31
Sulphur Dioxide SO, 64.06 1.24 78.7 430.6 36.05 40.00
Water Vapor HjO 18.02 1.33 221.2 647.8 33.31 34.07
(1) Approximate values based en average competition.
'Use straight line interpolation 01 ntrapolatlon to approximate C,. [In kJ/(kmol'K)] at actual Mot T. (For greater accuracy, average T should be used.)
Source: Ref. 56 with permission.
Copyright © 2003 by Taylor & Francis Group LLC