Page 36 - Chiral Separation Techniques
P. 36
1.3 Chromatographie Techniques 11
ensured the total retention of the chiral selector in the stationary phase [125]. The
separation of 2 g of the same leucine derivative employing the pH-zone refining
technique with the same instrument was later described [127]. (The elution pattern
of pH-zone-refining CCC bears a remarkable resemblance to that observed in dis-
placement chromatography and allows the displacement of ionizable molecules
through the CCC column by means of a pH gradient [116, 126].)
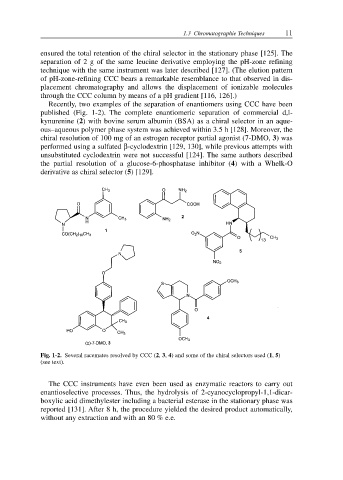
Recently, two examples of the separation of enantiomers using CCC have been
published (Fig. 1-2). The complete enantiomeric separation of commercial d,l-
kynurenine (2) with bovine serum albumin (BSA) as a chiral selector in an aque-
ous–aqueous polymer phase system was achieved within 3.5 h [128]. Moreover, the
chiral resolution of 100 mg of an estrogen receptor partial agonist (7-DMO, 3) was
performed using a sulfated β-cyclodextrin [129, 130], while previous attempts with
unsubstituted cyclodextrin were not successful [124]. The same authors described
the partial resolution of a glucose-6-phosphatase inhibitor (4) with a Whelk-O
derivative as chiral selector (5) [129].
Fig. 1-2. Several racemates resolved by CCC (2, 3, 4) and some of the chiral selectors used (1, 5)
(see text).
The CCC instruments have even been used as enzymatic reactors to carry out
enantioselective processes. Thus, the hydrolysis of 2-cyanocyclopropyl-1,1-dicar-
boxylic acid dimethylester including a bacterial esterase in the stationary phase was
reported [131]. After 8 h, the procedure yielded the desired product automatically,
without any extraction and with an 80 % e.e.