Page 81 - Chiral Separation Techniques
P. 81
3.3 Chiral Selectors 57
effects of support chemistry, surface polarity, length and polarity of the tether, and
selector loading [14]. Our group also demonstrated that separation media based on
an organic polymer support provide enhanced enantioselectivities and reduced reten-
tion times when compared to analogous silica-based chiral stationary phases, mostly
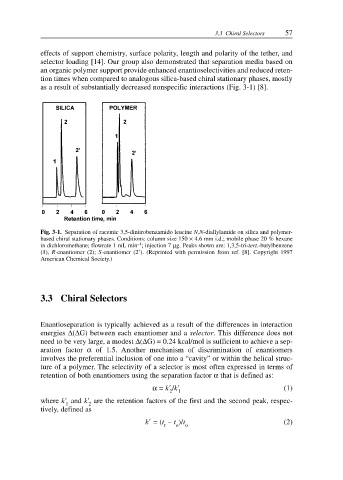
as a result of substantially decreased nonspecific interactions (Fig. 3-1) [8].
Fig. 3-1. Separation of racemic 3,5-dinitrobenzamido leucine N,N-diallylamide on silica and polymer-
based chiral stationary phases. Conditions: column size 150 × 4.6 mm i.d.; mobile phase 20 % hexane
–1
in dichloromethane; flowrate 1 mL min ; injection 7 µg. Peaks shown are: 1,3,5-tri-tert.-butylbenzene
(1), R-enantiomer (2); S-enantiomer (2 ). (Reprinted with permission from ref. [8]. Copyright 1997
American Chemical Society.)
3.3 Chiral Selectors
Enantioseparation is typically achieved as a result of the differences in interaction
energies ∆(∆G) between each enantiomer and a selector. This difference does not
need to be very large, a modest ∆(∆G) = 0.24 kcal/mol is sufficient to achieve a sep-
aration factor α of 1.5. Another mechanism of discrimination of enantiomers
involves the preferential inclusion of one into a “cavity” or within the helical struc-
ture of a polymer. The selectivity of a selector is most often expressed in terms of
retention of both enantiomers using the separation factor α that is defined as:
α = k /k (1)
2 1
where k and k are the retention factors of the first and the second peak, respec-
1 2
tively, defined as
k = (t – t )/t (2)
r o o