Page 402 - Corrosion Engineering Principles and Practice
P. 402
370 C h a p t e r 9 A t m o s p h e r i c C o r r o s i o n 371
tendency to form iron oxide. When it does resist corrosion it is due to
the formation of a thin film of protective iron oxide on its surface by
reaction with oxygen in the air. This film can prevent rusting in air at
99 percent RH, but a contaminant such as acid rain may destroy the
effectiveness of the film and permit continued corrosion. Thicker
films of iron oxide may act as protective coatings, and after the first
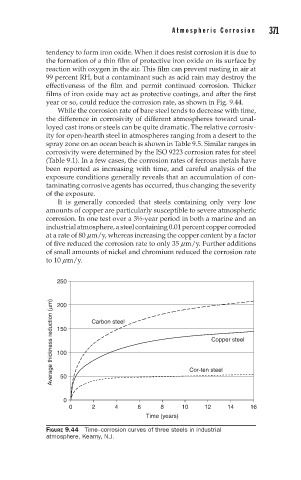
year or so, could reduce the corrosion rate, as shown in Fig. 9.44.
While the corrosion rate of bare steel tends to decrease with time,
the difference in corrosivity of different atmospheres toward unal-
loyed cast irons or steels can be quite dramatic. The relative corrosiv-
ity for open-hearth steel in atmospheres ranging from a desert to the
spray zone on an ocean beach is shown in Table 9.5. Similar ranges in
corrosivity were determined by the ISO 9223 corrosion rates for steel
(Table 9.1). In a few cases, the corrosion rates of ferrous metals have
been reported as increasing with time, and careful analysis of the
exposure conditions generally reveals that an accumulation of con-
taminating corrosive agents has occurred, thus changing the severity
of the exposure.
It is generally conceded that steels containing only very low
amounts of copper are particularly susceptible to severe atmospheric
corrosion. In one test over a 3½-year period in both a marine and an
industrial atmosphere, a steel containing 0.01 percent copper corroded
at a rate of 80 mm/y, whereas increasing the copper content by a factor
of five reduced the corrosion rate to only 35 mm/y. Further additions
of small amounts of nickel and chromium reduced the corrosion rate
to 10 mm/y.
250
Average thickness reduction (mm) 150 Carbon steel Cor-ten steel
200
Copper steel
100
50
0
0 2 4 6 8 10 12 14 16
Time (years)
FIGURE 9.44 Time–corrosion curves of three steels in industrial
atmosphere, Kearny, N.J.