Page 417 - Corrosion Engineering Principles and Practice
P. 417
386 C h a p t e r 1 0 C o r r o s i o n i n S o i l s a n d M i c r o b i o l o g i c a l l y I n f l u e n c e d C o r r o s i o n 387
Air
5
7, 8 Aluminum and 11
5
5
2 4, 5, 8 Polymers alloys
6
alloys Oil
Ferrous 2 1 Emulsions
2 Water 10 5 2, 5
6
5
Plastics
Soil 2 7, 8, 9
2 2 2, 5
Atomic H Hessian
Various metals
2, 3 5
2
Concrete Asphalt bitume
3
4
Protective coatings
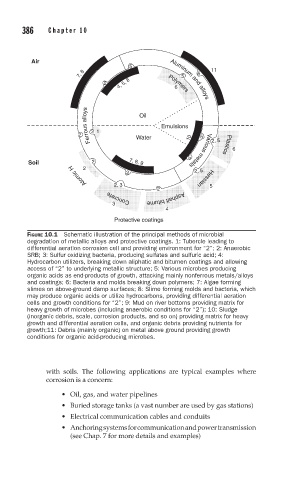
FIGURE 10.1 Schematic illustration of the principal methods of microbial
degradation of metallic alloys and protective coatings. 1: Tubercle leading to
differential aeration corrosion cell and providing environment for “2”; 2: Anaerobic
SRB; 3: Sulfur oxidizing bacteria, producing sulfates and sulfuric acid; 4:
Hydrocarbon utilizers, breaking down aliphatic and bitumen coatings and allowing
access of “2” to underlying metallic structure; 5: Various microbes producing
organic acids as end-products of growth, attacking mainly nonferrous metals/alloys
and coatings; 6: Bacteria and molds breaking down polymers; 7: Algae forming
slimes on above-ground damp surfaces; 8: Slime forming molds and bacteria, which
may produce organic acids or utilize hydrocarbons, providing differential aeration
cells and growth conditions for “2”; 9: Mud on river bottoms providing matrix for
heavy growth of microbes (including anaerobic conditions for “2”); 10: Sludge
(inorganic debris, scale, corrosion products, and so on) providing matrix for heavy
growth and differential aeration cells, and organic debris providing nutrients for
growth;11: Debris (mainly organic) on metal above ground providing growth
conditions for organic acid-producing microbes.
with soils. The following applications are typical examples where
corrosion is a concern:
• Oil, gas, and water pipelines
• Buried storage tanks (a vast number are used by gas stations)
• Electrical communication cables and conduits
• Anchoring systems for communication and power transmission
(see Chap. 7 for more details and examples)