Page 235 - Distillation theory
P. 235
P1: FCH/FFX P2: FCH/FFX QC: FCH/FFX T1: FCH
0521820928c06 CB644-Petlyuk-v1 June 11, 2004 20:17
6.9 Complexes of Heteroazeotropic and Heteroextractive Distillation 209
a) y D x L 1
2
x x
E
L 2
x
F s S
x
B 123 x
B
12
x x
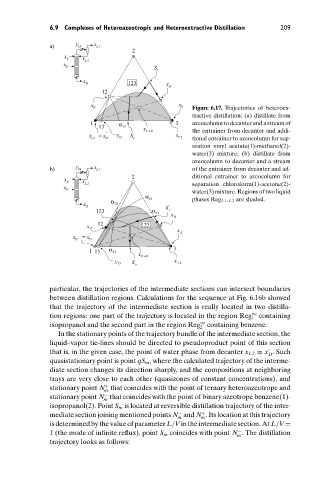
F E Figure 6.17. Trajectories of heteroex-
tractive distillation: (a) distillate from
1 α 3 azeocolumn to decanter and a stream of
13 12 x
x = x ' y S F +E x the entrainer from decanter and addi-
L 1 D D e L 2 tional entrainer to azeocolumn for sep-
aration vinyl acetate(1)-methanol(2)-
water(3) mixture; (b) distillate from
azeocolumn to decanter and a stream
b) y D x L 1 of the entrainer from decanter and ad-
2 ditional entrainer to azeocolumn for
x x
E
L 2 separation chloroform(1)-acetone(2)-
x
F water(3) mixture. Regions of two liquid
α
α 13 phases Reg L1–L2 are shaded.
x 23
B S
123 α s
12
x
B
12 123
x
F
x E
x = x D '
L
1
α 3
1 13 23
x
F +E
y S x
D e L 2
particular, the trajectories of the intermediate sections can intersect boundaries
between distillation regions. Calculations for the sequence at Fig. 6.16b showed
that the trajectory of the intermediate section is really located in two distilla-
tion regions: one part of the trajectory is located in the region Reg ∞ containing
1
isopropanol and the second part in the region Reg ∞ containing benzene.
2
In the stationary points of the trajectory bundle of the intermediate section, the
liquid–vapor tie-lines should be directed to pseudoproduct point of this section
that is, in the given case, the point of water phase from decanter x L2 ≡ x . Such
D
quasistationary point is point qS m , where the calculated trajectory of the interme-
diate section changes its direction sharply, and the compositions at neighboring
trays are very close to each other (quasizones of constant concentrations), and
stationary point N that coincides with the point of ternary heteroazeotrope and
+
m
stationary point N that coincides with the point of binary azeotrope benzene(1)-
−
m
isopropanol(2). Point S m is located at reversible distillation trajectory of the inter-
+
mediate section joining mentioned points N and N . Its location at this trajectory
−
m m
is determinedby the value of parameter L/Vin theintermediatesection.At L/V=
−
1 (the mode of infinite reflux), point S m coincides with point N . The distillation
m
trajectory looks as follows: