Page 74 - Earth's Climate Past and Future
P. 74
50 PART II • Tectonic-Scale Climate Change
How has this near-perfect balance been possible?
As we noted earlier, a thermostat can provide such a
balance. In our search for Earth’s thermostat within its 20
carbon system, we have ruled out volcanic input of
CO . The only other possibility left is chemical weath-
2 Temperature (°C) 10
ering. If the rate of chemical weathering is sensitive to
climate, it may be able to act as Earth’s thermostat.
0
Climatic Factors That Control Chemical
Weathering A 90°N 60° 30° 0° 30° 60° 90°S
Latitude
Decades of laboratory experiments and many field stud- 2000
ies have shown that rates of chemical weathering are
influenced by three environmental factors: tempera-
ture, precipitation, and vegetation. These factors all act
in a mutually reinforcing way to affect the intensity of 1000
chemical weathering. Precipitation (mm/yr)
Laboratory experiments have shown that higher
temperatures cause more rapid weathering of individual
silicate minerals. This trend is consistent with many 0
temperature-dependent chemical reactions in water B 90°N 60° 30° 0° 30° 60° 90°S
or other aqueous solutions. Weathering rates roughly Latitude
double for each 10°C increase in temperature. 3000
Unfortunately, it is difficult to transfer these labora-
tory results to studies of the real Earth. So far, experi-
ments have examined only a few of the many silicate
minerals that are common enough in Earth’s crust to be 2000
important contributors to the overall rate of silicate
weathering on a global scale. Natural chemical weather- Production (g/m 2 /yr)
ing rates are also difficult to determine in field studies 1000
because of the complicating effects from rapid carbonate
dissolution. Because dissolution occurs many times
faster than hydrolysis, the total amount of ions flowing 0
down rivers can easily be dominated by ions derived C –10 0 10 20 30
from limestone dissolution, which does not control CO Temperature (°C)
2
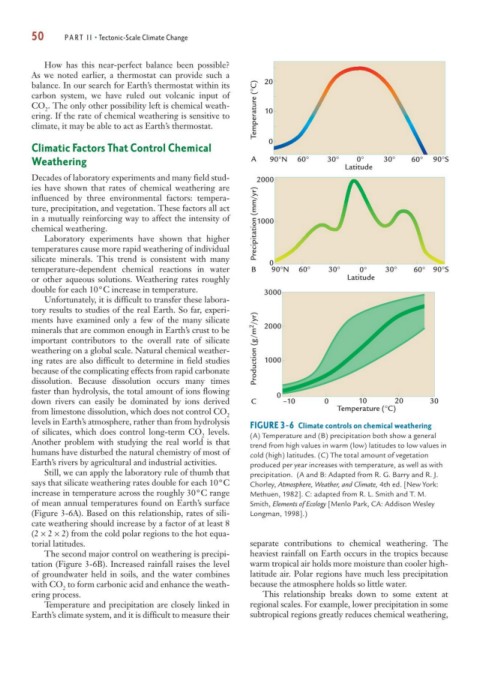
levels in Earth’s atmosphere, rather than from hydrolysis FIGURE 3-6 Climate controls on chemical weathering
of silicates, which does control long-term CO levels.
2 (A) Temperature and (B) precipitation both show a general
Another problem with studying the real world is that trend from high values in warm (low) latitudes to low values in
humans have disturbed the natural chemistry of most of cold (high) latitudes. (C) The total amount of vegetation
Earth’s rivers by agricultural and industrial activities. produced per year increases with temperature, as well as with
Still, we can apply the laboratory rule of thumb that precipitation. (A and B: Adapted from R. G. Barry and R. J.
says that silicate weathering rates double for each 10°C Chorley, Atmosphere, Weather, and Climate, 4th ed. [New York:
increase in temperature across the roughly 30°C range Methuen, 1982]. C: adapted from R. L. Smith and T. M.
of mean annual temperatures found on Earth’s surface Smith, Elements of Ecology [Menlo Park, CA: Addison Wesley
(Figure 3-6A). Based on this relationship, rates of sili- Longman, 1998].)
cate weathering should increase by a factor of at least 8
(2 × 2 × 2) from the cold polar regions to the hot equa-
torial latitudes. separate contributions to chemical weathering. The
The second major control on weathering is precipi- heaviest rainfall on Earth occurs in the tropics because
tation (Figure 3-6B). Increased rainfall raises the level warm tropical air holds more moisture than cooler high-
of groundwater held in soils, and the water combines latitude air. Polar regions have much less precipitation
with CO to form carbonic acid and enhance the weath- because the atmosphere holds so little water.
2
ering process. This relationship breaks down to some extent at
Temperature and precipitation are closely linked in regional scales. For example, lower precipitation in some
Earth’s climate system, and it is difficult to measure their subtropical regions greatly reduces chemical weathering,