Page 113 - Bruno Linder Elementary Physical Chemistry
P. 113
August 18, 2010 11:36 9in x 6in b985-ch09 Elementary Physical Chemistry
98 Elementary Physical Chemistry
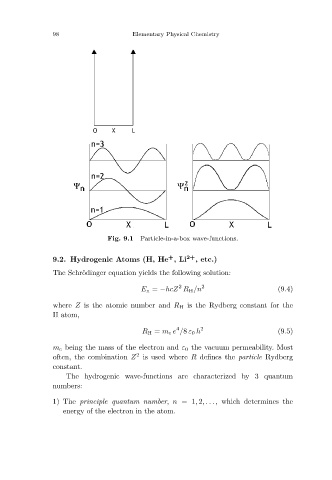
Particle-in-a-box wave-functions.
Fig. 9.1
+ + +
9.2. Hydrogenic Atoms (H, He ,Li 2+ + + ,etc.)
The Schr¨dinger equation yields the following solution:
o
2
E n = −hcZ R H /n 2 (9.4)
where Z is the atomic number and R H is the Rydberg constant for the
Hatom,
4
R H = m e e /8 ε 0 h 2 (9.5)
m e being the mass of the electron and ε 0 the vacuum permeability. Most
2
often, the combination Z is used where R defines the particle Rydberg
constant.
The hydrogenic wave-functions are characterized by 3 quantum
numbers:
1) The principle quantum number, n =1, 2,..., which determines the
energy of the electron in the atom.