Page 28 - Bruno Linder Elementary Physical Chemistry
P. 28
August 25, 2010 9:36 9in x 6in b985-ch02 Elementary Physical Chemistry
The First Law of Thermodynamics 13
2.4. Measurement of Work
Work is distance times force. Force is mass times acceleration.
The following are examples of work:
a) When lifting an object, a force must be applied in the direction away
from the earth, which is equal to the mass times the gravitational
acceleration, i.e. m × g. If the object moves a distance h,the work
done by the surrounding on the system is
w = h × mg (2.1)
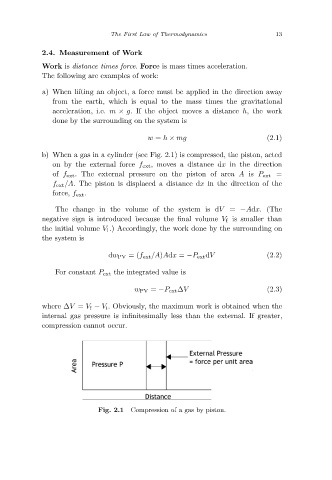
b) When a gas in a cylinder (see Fig. 2.1) is compressed, the piston, acted
on by the external force f ext ,moves a distance dx in the direction
of f ext. The external pressure on the piston of area A is P ext =
f ext /A. The piston is displaced a distance dx in the direction of the
force, f ext.
The change in the volume of the system is dV = −Adx.(The
negative sign is introduced because the final volume V f is smaller than
the initial volume V i .) Accordingly, the work done by the surrounding on
the system is
dw PV =(f ext/A)Adx = −P extdV (2.2)
For constant P ext the integrated value is
w PV = −P ext∆V (2.3)
where ∆V = V f − V i . Obviously, the maximum work is obtained when the
internal gas pressure is infinitesimally less than the external. If greater,
compression cannot occur.
Compression of a gas by piston.
Fig. 2.1