Page 236 - Elements of Chemical Reaction Engineering 3rd Edition
P. 236
208 Isothermal Reactor Design Chap. 4
while achieving not less than 90% conversion? Refemng to Table 1-1,
estimate the cost of the batch reactor.
(0 Write a couple of sentences describing what you learned from the prob-
lem and what you believe to be the point of the problem.
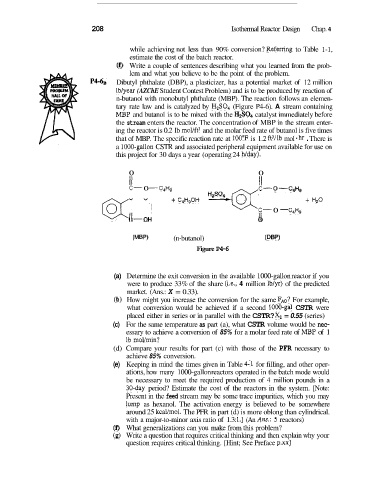
P4-6, Dibutyl phthalate (DBP), a plasticizer, has a potential market of 12 million
lblyear (AZChE Student Contest Problem) and is to be produced by reaction of
n-butanol with monobutyl phthalate (MBP). The reaction follows an elemen-
tary rate law and is catalyzed by H2S04 (Figure P4-6). A stream containing
MF3P and butanol is to be mixed with the HzSO4 catalyst immediately before
the stream enters the reactor. The concentration of MBP in the stream enter-
ing the reactor is 0.2 lb mol/ft3 and the molar feed rate of butanol is five times
that of MBP. The specific reaction rate at 100°F is 1.2 ft3/lb mol hr . There is
a lO00-gallon CSTR and associated peripheral equipment available for use on
this project for 30 days a year (operating 24 Wday).
0 0
II II
C- 0 - C4H9 C- O-C4H9
@LOH C- 0 - C4HQ + HZ0
+ C4H,0H
II
I1
0
0
(MBP) (n-butanol) (DBP)
Figure P4-6
(a) Determine the exit conversion in the available 1000-gallon reactor if you
were to produce 33% of the share (Le., 4 million lb/yr) of the predicted
market. (Ans.: X = 0.33).
(b) How might you increase the conversion for the same Fm? For example,
what conversion would be achieved if a second 1OOO-gal CSTR were
placed either in series or in parallel with the CSTR? X2 = 0.55 (series)
(c) For the same temperature as part (a), what CSTR volume would be nec-
essary to achieve a conversion of 85% for a molar feed rate of h4BP of 1
ib moYmin?
(d) Compare your results for part (c) with those of the PFR necessary to
achieve 85% conversion.
(e) Keeping in mind the times given in Table 4--1 for filling, and other oper-
ations, how many 1000-gallon reactors operated in the batch mode would
be necessary to meet the required production of 4 million pounds in a
3Oday period? Estimate the cost of the reactors in the system. [Note:
Present in the feed stream may be some trace impurities, which you may
lump as hexanol. The activation energy is believed to be somewhere
around 25 kcal/mol. The PFR in part (d) is more oblong than cylindrical.
with a major-to-minor axis ratio of 1.3: I.] (An Am.: 5 reactors)
(0 What generalizations can you make from this problem?
(g) Write a question that requires critical thinking and then explain why your
question requires critical thinking. [Hint; See Preface pxx]