Page 322 - Elements of Chemical Reaction Engineering 3rd Edition
P. 322
294 Multiple Reactions Chap. 6
3. Optimum yield. The concentration of B goes through a maximum at a point
along the reactor. Consequently, to find the optimum reactor length, we need
to differentiate Equation (E6-3.7):
(E6-388)
Solving for 7LPt gives
(E6-3.9)
(E6-3.10)
The corresponding conversion of A is
(E6-3.11)
The yield has been defined as
moles of acetaldehyde in exit
= moles of ethanol fed
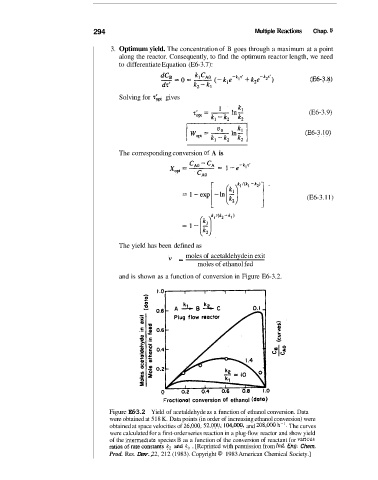
and is shown as a function of conversion in Figure E6-3.2.
-
Froctionol conversion of ethanol (dolo)
Figure E6-3.2 Yield of acetaldehyde as a function of ethanol conversion. Data
were obtained at 518 K. Data points (in order of increasing ethanol conversion) were
obtained at space velocities of 26,000, 52,000, 104,oOO, and 208,000 h-I. The curves
were calculated for a first-order series reaction in a plug-flow reactor and show yield
of the intermediaKt? species B as a function of the conversion of reactant for various
ratios of rate constants k2 ad k, . [Reprinted with permission from Ind. En& Chem.
Prod. Res. Dev., 22, 212 (1983). Copyright 0 1983 American Chemical Society.]