Page 329 - Elements of Chemical Reaction Engineering Ebook
P. 329
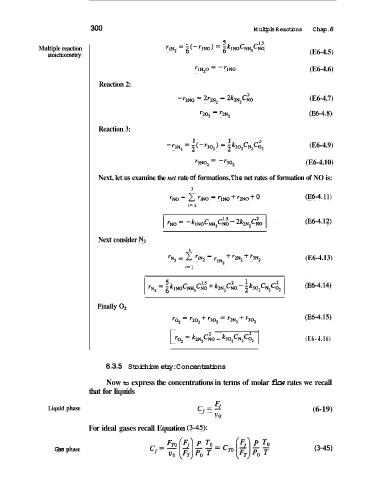
300 Multiple Reactions Chap. 6
=
Multiple reaction ' 1 ~ ~5 ,(-.]NO> = ;km$&,Ci% (E6-4.5)
stoichiomeq
rlHZO = -TINO (E6-4.6)
Reaction 2:
-'2NO = 2r2N2 = 2k2N2c& (E6-4.7)
*20, = '2N2 (E6-4.8)
Reaction 3:
1 1 2
-'3N2 = 2(-'302) = ~k302cN2c02 (E6-4.9)
'3N02 = -'302 (E6-4.10)
Next, let us examine the net rate sf formations. The net rates of formation of NO is:
3
'NO = 1 = rlNo + '2NO + (E64 11)
'NO
i= 1
Next consider Nz
3
'N2 c 'iN2 = + '2N2 + r3N2 (E6-4.13)
i= 1
FiZNlc~O k302cN2c62 I (E6-4.16)
6.3.5 Stoichiometry: Concentrations
Now to express the concentrations in terms of molar flow rates we recall
that for liquids
F.
Liquid phase ci = 1 (6-19)
UO
For ideal gases recall Equation (3-45):
Gas phase (3-45)