Page 390 - Elements of Chemical Reaction Engineering Ebook
P. 390
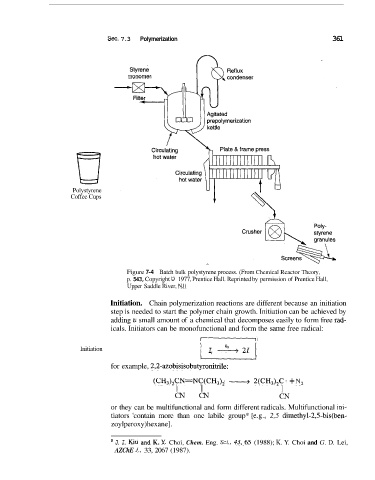
Sec. 7.3 Polymerization 361
inonomer
Polystyrene
Coffee Cups
Figure 7-4 Batch bulk polystyrene process. (From Chemical Reactor Theory,
p. 543, Copyright 0 1977, Prentice Hall. Reprinted by permission of Prentice Hall,
Upper Saddle River, NJ)
Initiation. Chain polymerization reactions are different because an initiation
step is needed to start the polymer chain growth. Initiation can be achieved by
adding a small amount of a chemical that decomposes easily to form free r(ad-
icals. Initiators can be monofunctional and form the same free radical:
1 I, A 21 1
I'
Initiation
I
for example, 2,2-aczobisisobutyronitrile:
(CH,),CN=NC(CH3), 2(CH,),C. + N,
I I I
CN CN CN
or they can be multifunctional and form different radicals. Multifunctional imi-
tiators 'contain more than one labile group* [e.g., 2,5 dimethyl-2,5-bis(be:n-
zoylperoxy )hexane].
* J. J. Kiu and K. Y. Choi, Chem. Eng. Sci., 43,.65 (1988); K. Y. Choi and G. D. Lei,
AZChE J., 33, 2067 (1987).