Page 82 - Encyclopedia of Chemical Compounds 3 Vols
P. 82
H O
H C C
C C OH
C C
H C O
H C CH 3
O
OTHER NAMES:
Aspirin; see Overview
for more names
FORMULA:
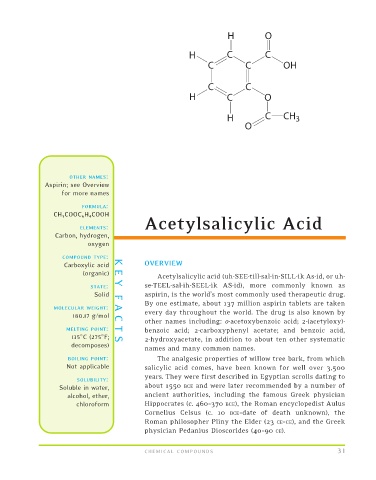
CH 3 COOC 6 H 4 COOH
Acetylsalicylic Acid
ELEMENTS:
Carbon, hydrogen,
oxygen
Carboxylic acid OVERVIEW
(organic)
Acetylsalicylic acid (uh-SEE-till-sal-in-SILL-ik As-id, or uh-
COMPOUND TYPE: KE
STATE: Y se-TEEL-sal-ih-SEEL-ik AS-id), more commonly known as
Solid F aspirin, is the world’s most commonly used therapeutic drug.
By one estimate, about 137 million aspirin tablets are taken
MOLECULAR WEIGHT: A
every day throughout the world. The drug is also known by
180.17 g/mol C
other names including: o-acetoxybenzoic acid; 2-(acetyloxy)-
MELTING POINT: T benzoic acid; 2-carboxyphenyl acetate; and benzoic acid,
135 C (275 F; 2-hydroxyacetate, in addition to about ten other systematic
S
decomposes)
names and many common names.
BOILING POINT: The analgesic properties of willow tree bark, from which
Not applicable salicylic acid comes, have been known for well over 3,500
years. They were first described in Egyptian scrolls dating to
SOLUBILITY:
Soluble in water, about 1550 BCE and were later recommended by a number of
alcohol, ether, ancient authorities, including the famous Greek physician
chloroform Hippocrates (c. 460–370 BCE), the Roman encyclopedist Aulus
Cornelius Celsus (c. 10 BCE–date of death unknown), the
Roman philosopher Pliny the Elder (23 CE–CE), and the Greek
physician Pedanius Dioscorides (40–90 CE).
CHEMICAL COMPOUNDS 31