Page 258 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 258
P1: GLQ/GLE P2: GPB Final Pages
Encyclopedia of Physical Science and Technology EN014J-683 July 30, 2001 20:3
666 Separation and Purification of Biochemicals
of the respective substances in relation to the isotherm
and concentration of the displacer. The concentration of
the components may thus be increased in respect to their
concentration in the feed. This feature is of interest not
only in preparative scale separations, but also for enrich-
ment of certain components in trace analyses. The effect of
feed and displacer concentrations, bed length, and mass-
transfer effects on the separation have been treated theo-
retically, and in many cases the results have experimental
support.
Although further work is needed to exploit the full po-
tential of displacement biochromatography, results accu-
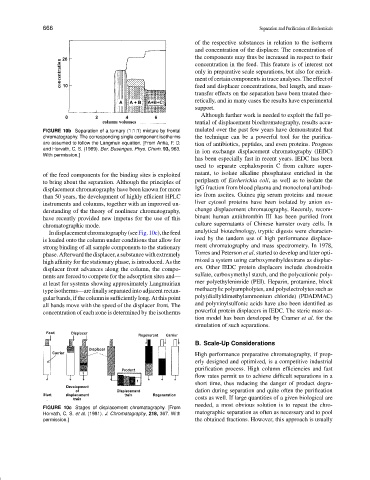
FIGURE 10b Separation of a ternary (1:1:1) mixture by frontal mulated over the past few years have demonstrated that
chromatography. The corresponding single component isotherms the technique can be a powerful tool for the purifica-
are assumed to follow the Langmuir equation. [From Antia, F. D. tion of antibiotics, peptides, and even proteins. Progress
and Horvath, C. S. (1989). Ber. Busenges. Phys. Chem. 93, 963.
in ion exchange displacement chromatography (IEDC)
With permission.]
has been especially fast in recent years. IEDC has been
used to separate cephalosporin C from culture super-
of the feed components for the binding sites is exploited natant, to isolate alkaline phosphatase enriched in the
to bring about the separation. Although the principles of periplasm of Escherichia coli, as well as to isolate the
displacement chromatography have been known for more IgG fraction from blood plasma and monoclonal antibod-
than 50 years, the development of highly efficient HPLC ies from ascites. Guinea pig serum proteins and mouse
instruments and columns, together with an improved un- liver cytosol proteins have been isolated by anion ex-
derstanding of the theory of nonlinear chromatography, change displacement chromatography. Recently, recom-
have recently provided new impetus for the use of this binant human antithrombin III has been purified from
chromatographic mode. culture supernatants of Chinese hamster ovary cells. In
Indisplacementchromatography(seeFig.10c),thefeed analytical biotechnology, tryptic digests were character-
is loaded onto the column under conditions that allow for ized by the tandem use of high performance displace-
strong binding of all sample components to the stationary ment chromatography and mass spectrometry. In 1978,
phase.Afterwardthedisplacer,asubstancewithextremely Torres and Peterson et al. started to develop and later opti-
high affinity for the stationary phase, is introduced. As the mized a system using carboxymethyldextrans as displac-
displacer front advances along the column, the compo- ers. Other IEDC protein displacers include chondroitin
nents are forced to compete for the adsorption sites and— sulfate, carboxymethyl starch, and the polycationic poly-
at least for systems showing approximately Langmuirian mer polyethylenimide (PEI). Heparin, protamine, block
type isotherms—are finally separated into adjacent rectan- methacrylic polyampholytes, and polyelectrolytes such as
gular bands, if the column is sufficiently long. At this point poly(diallyldimethylammonium chloride) (PDADMAC)
all bands move with the speed of the displacer front. The and polyvinylsulfonic acids have also been identified as
concentration of each zone is determined by the isotherms powerful protein displacers in IEDC. The steric mass ac-
tion model has been developed by Cramer et al. for the
simulation of such separations.
B. Scale-Up Considerations
High performance preparative chromatography, if prop-
erly designed and optimized, is a competitive industrial
purification process. High column efficiencies and fast
flow rates permit us to achieve difficult separations in a
short time, thus reducing the danger of product degra-
dation during separation and quite often the purification
costs as well. If large quantities of a given biological are
needed, a most obvious solution is to repeat the chro-
FIGURE 10c Stages of displacement chromatography. [From
Horvath, C. S. et al. (1981). J. Chromatography, 218, 367. With matographic separation as often as necessary and to pool
permission.] the obtained fractions. However, this approach is usually