Page 357 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 357
P1: GLQ Final Pages
Encyclopedia of Physical Science and Technology EN009K-419 July 19, 2001 20:57
292 Membranes, Synthetic, Applications
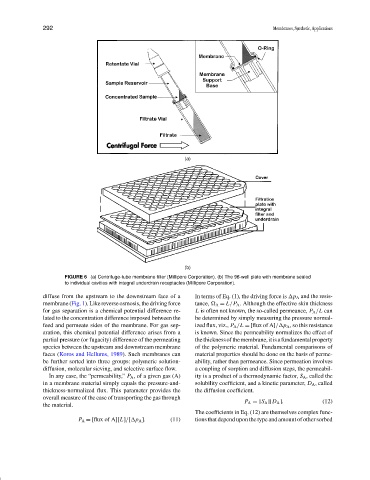
(a)
(b)
FIGURE 6 (a) Centrifuge-tube membrane filter (Millipore Corporation). (b) The 96-well plate with membrane sealed
to individual cavities with integral underdrain receptacles (Millipore Corporation).
diffuse from the upstream to the downstream face of a In terms of Eq. (1), the driving force is
p A and the resis-
membrane (Fig. 1). Like reverse osmosis, the driving force tance, A = L/P A . Although the effective skin thickness
for gas separation is a chemical potential difference re- L is often not known, the so-called permeance, P A /L can
lated to the concentration difference imposed between the be determined by simply measuring the pressure normal-
feed and permeate sides of the membrane. For gas sep- ized flux, viz., P A /L = [flux of A]/
p A , so this resistance
aration, this chemical potential difference arises from a is known. Since the permeability normalizes the effect of
partial pressure (or fugacity) difference of the permeating thethicknessofthemembrane,itisafundamentalproperty
species between the upstream and downstream membrane of the polymeric material. Fundamental comparisons of
faces (Koros and Hellums, 1989). Such membranes can material properties should be done on the basis of perme-
be further sorted into three groups: polymeric solution- ability, rather than permeance. Since permeation involves
diffusion, molecular sieving, and selective surface flow. a coupling of sorption and diffusion steps, the permeabil-
In any case, the “permeability,” P A , of a given gas (A) ity is a product of a thermodynamic factor, S A , called the
in a membrane material simply equals the pressure-and- solubility coefficient, and a kinetic parameter, D A , called
thickness-normalized flux. This parameter provides the the diffusion coefficient.
overall measure of the ease of transporting the gas through
P A = [S A ][D A ]. (12)
the material.
The coefficients in Eq. (12) are themselves complex func-
P A = [flux of A][L]/[
p A ]. (11) tions that depend upon the type and amount of other sorbed