Page 53 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 53
P1: FWD Revised Pages
Encyclopedia of Physical Science and Technology En001c-14 May 7, 2001 18:25
276 Aerosols
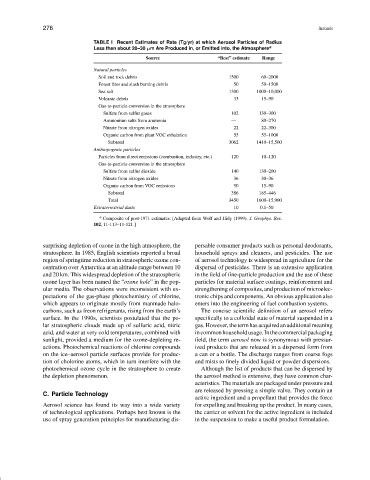
TABLE I Recent Estimates of Rate (Tg/yr) at which Aerosol Particles of Radius
Less than about 20–30 µm Are Produced in, or Emitted into, the Atmosphere a
Source “Best” estimate Range
Natural particles
Soil and rock debris 1500 60–2000
Forest fires and slash burning debris 50 50–1500
Sea salt 1300 1000–10,000
Volcanic debris 33 15–90
Gas-to-particle conversion in the atmosphere
Sulfate from sulfur gases 102 130–300
Ammonium salts from ammonia — 80–270
Nitrate from nitrogen oxides 22 22–300
Organic carbon from plant VOC exhalation 55 55–1000
Subtotal 3062 1410–15,500
Anthropogenic particles
Particles from direct emissions (combustion, industry, etc.) 120 10–120
Gas-to-particle conversion in the atmosphere
Sulfate from sulfur dioxide 140 130–200
Nitrate from nitrogen oxides 36 30–36
Organic carbon from VOC emissions 90 15–90
Subtotal 386 185–446
Total 3450 1600–15,900
Extraterrestrial dusts 10 0.1–50
a Composite of post-1971 estimates. [Adapted from Wolf and Hidy (1999). J. Geophys. Res.
102, 11-113–11-121.]
surprising depletion of ozone in the high atmosphere, the persable consumer products such as personal deodorants,
stratosphere. In 1985, English scientists reported a broad household sprays and cleaners, and pesticides. The use
region of springtime reduction in stratospheric ozone con- of aerosol technology is widespread in agriculture for the
centration over Antarctica at an altitude range between 10 dispersal of pesticides. There is an extensive application
and 20 km. This widespread depletion of the stratospheric in the field of fine-particle production and the use of these
ozone layer has been named the “ozone hole” in the pop- particles for material surface coatings, reinforcement and
ular media. The observations were inconsistent with ex- strengtheningofcomposites,andproductionofmicroelec-
pectations of the gas-phase photochemistry of chlorine, tronic chips and components. An obvious application also
which appears to originate mostly from manmade halo- enters into the engineering of fuel combustion systems.
carbons, such as freon refrigerants, rising from the earth’s The concise scientificdefinition of an aerosol refers
surface. In the 1990s, scientists postulated that the po- specifically to a colloidal state of material suspended in a
lar stratospheric clouds made up of sulfuric acid, nitric gas.However,thetermhasacquiredanadditionalmeaning
acid, and water at very cold temperatures, combined with incommonhouseholdusage.Inthecommercialpackaging
sunlight, provided a medium for the ozone-depleting re- field, the term aerosol now is synonymous with pressur-
actions. Photochemical reactions of chlorine compounds ized products that are released in a dispersed form from
on the ice–aerosol particle surfaces provide for produc- a can or a bottle. The discharge ranges from coarse fogs
tion of cholorine atoms, which in turn interfere with the and mists to finely divided liquid or powder dispersions.
photochemical ozone cycle in the stratosphere to create Although the list of products that can be dispersed by
the depletion phenomenon. the aerosol method is extensive, they have common char-
acteristics. The materials are packaged under pressure and
are released by pressing a simple valve. They contain an
C. Particle Technology
active ingredient and a propellant that provides the force
Aerosol science has found its way into a wide variety for expelling and breaking up the product. In many cases,
of technological applications. Perhaps best known is the the carrier or solvent for the active ingredient is included
use of spray generation principles for manufacturing dis- in the suspension to make a useful product formulation.