Page 315 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 315
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009N-447 July 19, 2001 23:3
832 Microwave Molecular Spectroscopy
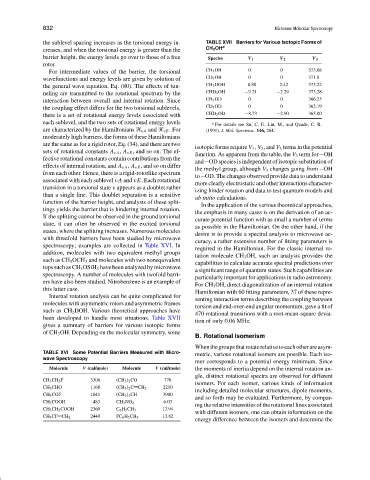
the sublevel spacing increases as the torsional energy in- TABLE XVII Barriers for Various Isotopic Forms of
creases, and when the torsional energy is greater than the CH 3 OH a
barrier height, the energy levels go over to those of a free Species V 1 V 2 V 3
rotor.
For intermediate values of the barrier, the torsional CH 3 OH 0 0 373.08
wavefunctions and energy levels are given by solution of CD 3 OH 0 0 371.8
the general wave equation, Eq. (80). The effects of tun- CH 2 DOH 8.80 2.42 373.22
neling are transmitted to the rotational spectrum by the CHD 2 OH −9.21 −2.29 373.28
interaction between overall and internal rotation. Since CH 3 OD 0 0 366.25
the coupling effect differs for the two torsional sublevels, CD 3 OD 0 0 362.19
there is a set of rotational energy levels associated with CHD 2 OD −8.73 −2.90 365.00
each sublevel, and the two sets of rotational energy levels a For details see Su, C. F., Liu, M., and Quade, C. R.
are characterized by the Hamiltonians vA and vE .For (1991). J. Mol. Spectrosc. 146, 264.
moderately high barriers, the forms of these Hamiltonians
are the same as for a rigid rotor, Eq. (34), and there are two
isotopic forms require V 1 , V 2 , and V 3 terms in the potential
sets of rotational constants A vA , A vE , and so on. The ef-
function. As apparent from the table, the V 3 term for OH
fective rotational constants contain contributions from the
and OD species is independent of isotopic substitution of
effects of internal rotation, and A vA , A vE , and so on differ
the methyl group, although V 3 changes going from OH
from each other. Hence, there is a rigid-rotorlike spectrum
to OD. The changes observed provide data to understand
associated with each sublevel vA and vE. Each rotational
more clearly electrostatic and other interactions character-
transition in a torsional state v appears as a doublet rather
izing hinder rotation and data to test quantum models and
than a single line. This doublet separation is a sensitive
ab initio calculations.
function of the barrier height, and analysis of these split-
In the application of the various theoretical approaches,
tings yields the barrier that is hindering internal rotation.
the emphasis in many cases is on the derivation of an ac-
If the splitting cannot be observed in the ground torsional
curate potential function with as small a number of terms
state, it can often be observed in the excited torsional
as possible in the Hamiltonian. On the other hand, if the
states, where the splitting increases. Numerous molecules
desire is to provide a spectral analysis to microwave ac-
with threefold barriers have been studied by microwave
curacy, a rather extensive number of fitting parameters is
spectroscopy; examples are collected in Table XVI. In
required in the Hamiltonian. For the classic internal ro-
addition, molecules with two equivalent methyl groups
tation molecule CH 3 OH, such an analysis provides the
such as CH 3 OCH 3 and molecules with two nonequivalent
capabilities to calculate accurate spectral predictions over
tops such as CH 3 OSiH 3 have been analyzed by microwave
a significant range of quantum states. Such capabilities are
spectroscopy. A number of molecules with twofold barri-
particularly important for applications in radio astronomy.
ers have also been studied. Nitrobenzene is an example of
For CH 3 OH, direct diagonalization of an internal rotation
this latter case.
Hamiltonian with 60 fitting parameters, 37 of these repre-
Internal rotation analysis can be quite complicated for
senting interaction terms describing the coupling between
molecules with asymmetric rotors and asymmetric frames
torsion and end-over-end angular momentum, gave a fitof
such as CH 2 DOH. Various theoretical approaches have
470 rotational transitions with a root-mean-square devia-
been developed to handle most situations. Table XVII
tion of only 0.06 MHz.
gives a summary of barriers for various isotopic forms
of CH 3 OH. Depending on the molecular symmetry, some
B. Rotational Isomerism
Whenthegroupsthatrotaterelativetoeachotherareasym-
TABLE XVI Some Potential Barriers Measured with Micro- metric, various rotational isomers are possible. Each iso-
wave Spectroscopy
mer corresponds to a potential energy minimum. Since
Molecule V (cal/mole) Molecule V (cal/mole) the moments of inertia depend on the internal rotation an-
gle, distinct rotational spectra are observed for different
CH 3 CH 2 F 3306 (CH 3 ) 2 CO 778
isomers. For each isomer, various kinds of information
CH 3 CHO 1168 (CH 3 ) 2 C CH 2 2210
including detailed molecular structures, dipole moments,
CH 3 COF 1041 (CH 3 ) 3 CH 3900
and so forth may be evaluated. Furthermore, by compar-
CH 3 COOH 483 CH 3 NO 2 6.03
ing the relative intensities of the rotational lines associated
CH 3 CH 2 COOH 2360 C 6 H 5 CH 3 13.94
with different isomers, one can obtain information on the
CH 3 CF CH 2 2440 FC 6 H 5 CH 3 13.82
energy difference between the isomers and determine the