Page 168 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 168
P1: GKY/MBQ P2: GLQ Final Pages
Encyclopedia of Physical Science and Technology en012i-947 July 26, 2001 11:11
676 Polymers, Inorganic and Organometallic
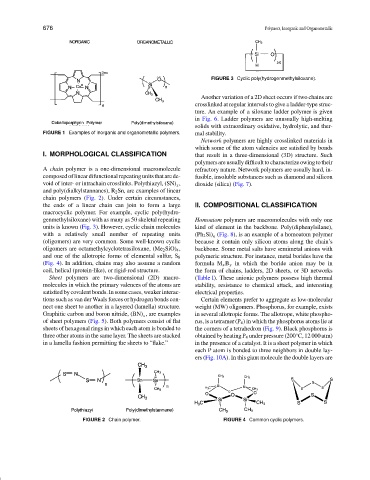
FIGURE 3 Cyclic poly(hydrogenmethylsiloxane).
Another variation of a 2D sheet occurs if two chains are
crosslinked at regular intervals to give a ladder-type struc-
ture. An example of a siloxane ladder polymer is given
in Fig. 6. Ladder polymers are unusually high-melting
solids with extraordinary oxidative, hydrolytic, and ther-
FIGURE 1 Examples of inorganic and organometallic polymers. mal stability.
Network polymers are highly crosslinked materials in
which some of the atom valencies are satisfied by bonds
I. MORPHOLOGICAL CLASSIFICATION that result in a three-dimensional (3D) structure. Such
polymersareusuallydifficulttocharacterizeowingtotheir
A chain polymer is a one-dimensional macromolecule refractory nature. Network polymers are usually hard, in-
composedoflineardifunctionalrepeatingunitsthatarede- fusible, insoluble substances such as diamond and silicon
void of inter- or intrachain crosslinks. Polythiazyl, (SN) , dioxide (silica) (Fig. 7).
x
and poly(dialkylstannanes), R 2 Sn, are examples of linear
chain polymers (Fig. 2). Under certain circumstances,
the ends of a linear chain can join to form a large II. COMPOSITIONAL CLASSIFICATION
macrocyclic polymer. For example, cyclic poly(hydro-
genmethylsiloxane) with as many as 50 skeletal repeating Homoatom polymers are macromolecules with only one
units is known (Fig. 3). However, cyclic chain molecules kind of element in the backbone. Poly(diphenylsilane),
with a relatively small number of repeating units (Ph 2 Si) (Fig. 8), is an example of a homoatom polymer
n
(oligomers) are very common. Some well-known cyclic because it contain only silicon atoms along the chain’s
oligomers are octamethylcyclotetrasiloxane, (Me 2 SiO) 4 , backbone. Some metal salts have semimetal anions with
polymeric structure. For instance, metal borides have the
and one of the allotropic forms of elemental sulfur, S 8
(Fig. 4). In addition, chains may also assume a random formula M x B y in which the boride anion may be in
coil, helical (protein-like), or rigid-rod structure. the form of chains, ladders, 2D sheets, or 3D networks
Sheet polymers are two-dimensional (2D) macro- (Table I). These anionic polymers possess high thermal
molecules in which the primary valences of the atoms are stability, resistance to chemical attack, and interesting
satisfied by covalent bonds. In some cases, weaker interac- electrical properties.
tions such as van der Waals forces or hydrogen bonds con- Certain elements prefer to aggregate as low-molecular
nect one sheet to another in a layered (lamella) structure. weight (MW) oligomers. Phosphorus, for example, exists
Graphitic carbon and boron nitride, (BN) , are examples in several allotropic forms. The allotrope, white phospho-
x
of sheet polymers (Fig. 5). Both polymers consist of flat rus, is a tetramer (P 4 ) in which the phosphorus atoms lie at
sheets of hexagonal rings in which each atom is bonded to the corners of a tetrahedron (Fig. 9). Black phosphorus is
◦
three other atoms in the same layer. The sheets are stacked obtained by heating P 4 under pressure (200 C,12 000atm)
in a lamella fashion permitting the sheets to “flake.” in the presence of a catalyst. It is a sheet polymer in which
each P atom is bonded to three neighbors in double lay-
ers (Fig. 10A). In this giant molecule the double layers are
FIGURE 2 Chain polymer. FIGURE 4 Common cyclic polymers.