Page 107 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 107
P1: GPB/GJP P2: FYK Final Pages
Encyclopedia of Physical Science and Technology EN005F-213 June 15, 2001 20:32
Electron Transfer Reactions 349
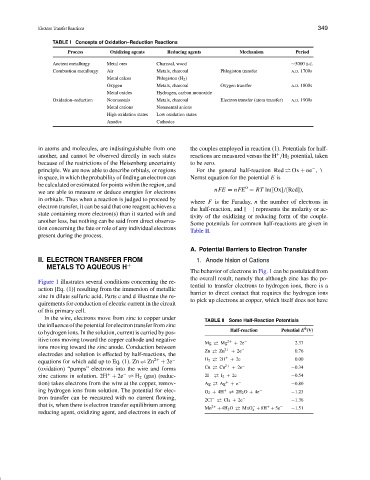
TABLE I Concepts of Oxidation–Reduction Reactions
Process Oxidizing agents Reducing agents Mechanism Period
Ancient metallurgy Metal ores Charcoal, wood ∼5000 B.C.
Combustion metallurgy Air Metals, charcoal Phlogiston transfer A.D. 1700s
Metal calces Phlogiston (H 2 )
Oxygen Metals, charcoal Oxygen transfer A.D. 1800s
Metal oxides Hydrogen, carbon monoxide
Oxidation–reduction Nonmentals Metals, charcoal Electron transfer (atom transfer) A.D. 1900s
Metal cations Nonmental anions
High oxidation states Low oxidation states
Anodes Cathodes
in atoms and molecules, are indistinguishable from one the couples employed in reaction (1). Potentials for half-
+
another, and cannot be observed directly in such states reactions are measured versus the H /H 2 potential, taken
because of the restrictions of the Heisenberg uncertainty to be zero.
principle. We are now able to describe orbitals, or regions For the general half-reaction Red Ox + ne , \
−
inspace,inwhichtheprobabilityoffindinganelectroncan Nernst equation for the potential E is
be calculated or estimated for points within the region, and 0
nFE = nFE − RT ln([Ox]/[Red]),
we are able to measure or deduce energies for electrons
in orbitals. Thus when a reaction is judged to proceed by
where F is the Faraday, n the number of electrons in
electron transfer, it can be said that one reagent achieves a
the half-reaction, and [ ] represents the molarity or ac-
state containing more electron(s) than it started with and
tivity of the oxidizing or reducing form of the couple.
another less, but nothing can be said from direct observa-
Some potentials for common half-reactions are given in
tion concerning the fate or role of any individual electrons
Table II.
present during the process.
A. Potential Barriers to Electron Transfer
II. ELECTRON TRANSFER FROM 1. Anode hlsion of Cations
METALS TO AQUEOUS H +
The behavior of electrons in Fig. 1 can be postulated from
the overall result, namely that although zinc has the po-
Figure 1 illustrates several conditions concerning the re-
tential to transfer electrons to hydrogen ions, there is a
action [Eq. (1)] resulting from the immersion of metallic
barrier to direct contact that requires the hydrogen ions
zinc in dilute sulfuric acid. Parts c and d illustrate the re-
to pick up electrons at copper, which itself does not have
quirements for conduction of electric current in the circuit
of this primary cell.
In the wire, electrons move from zinc to copper under TABLE II Some Half-Reaction Potentials
theinfluenceofthepotentialforelectrontransferfromzinc
0
Half-reaction Potential E (V)
to hydrogen ions. In the solution, current is carried by pos-
itive ions moving toward the copper cathode and negative
Mg → Mg 2+ + 2e − 2.37
←
ions moving toward the zinc anode. Conduction between
Zn → Zn 2+ + 2e − 0.76
←
electrodes and solution is effected by half-reactions, the + −
→
equations for which add up to Eq. (1). Zn Zn 2+ + 2e − H 2 ← 2H + 2e 0.00
(oxidation) “pumps” electrons into the wire and forms Cu → Cu 2+ + 2e − −0.34
←
zinc cations in solution. 2H + 2e H 2 (gas) (reduc- 2I − → I 2 + 2e − −0.54
−
+
←
tion) takes electrons from the wire at the copper, remov- Ag → Ag + + e − −0.80
←
ing hydrogen ions from solution. The potential for elec- O 2 + 4H + → 2H 2 O + 4e − −1.23
←
tron transfer can be measured with no current flowing,
2Cl − → Cl 2 + 2e − −1.36
←
that is, when there is electron transfer equilibrium among
+
Mn 2+ + 4H 2 O → MnO + 8H + 5e − −1.51
−
←
4
reducing agent, oxidizing agent, and electrons in each of