Page 139 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 139
P1: GLM Final Pages
Encyclopedia of Physical Science and Technology EN007C-307 June 29, 2001 19:40
216 Halogen Chemistry
Substitution of hydrogen by the halogen atom results in to that expected from the inductive effect. The resonance
an increase in the boiling point, melting point, and den- effect alters the chemical reactivity of the molecule in two
sity of the organic compound. (Fluorocarbons containing ways. First, the partially positive halogen atom is much
up to four carbon atoms boil somewhat higher than their less susceptible to nucleophilic attack than in saturated
corresponding hydrocarbons, but the reverse is true of flu- halides [Eq. (54)]. Second, the partially negative carbon
orocarbons with more than four carbons.) This increase is in the multiple bond becomes more susceptible to attack
greater the heavier the halogen and, in general, the larger by reagents that are electron-poor (eletrophilic).
the number of halogen atoms present. However, in poly- Equation (56) shows the addition of hydrogen bromide
halogenated compounds, the positions of the halogens rel- to vinyl bromide where the direction of addition is con-
ative to each other are also an important consideration. If trolled by the resonance effect. The positive hydrogen,
+
this molecular orientation is symmetrical, a substantial H , adds to the partially negative carbon on the double
decrease in the melting and boiling points may result. bond, while the negative bromide ion adds to the carbon
When halogens are present, they occupy terminal posi- that is already halogenated:
tions on the carbon chain and modify the reactivity of a
portion of the molecule in their immediate vicinity. Since
(56)
carbon is less electronegative than any of the halogens,
the shared pair of electrons in the C X bond is displaced
The situation is more complex when halogens are present
or polarized towards the halogen. For saturated carbons
in extensively unsaturated or aromatic ring systems. If
(carbons with only single bonds), the result of the induc-
only one halogen is bound directly to a benzene ring, in-
tive effect associated with this difference in electronega-
teraction of the halogen electrons with the multiple bonds
tivities is to make the halogen partially negative and the
on the ring produces partial negative charges on the ring
halogenated carbon partially positive. The carbon atom
carbons that are ortho and para to the halogen atom. The
then becomes susceptible to attack by reagents which are
charge movement resulting from this resonance effect is
electron-rich (nucleophilic).
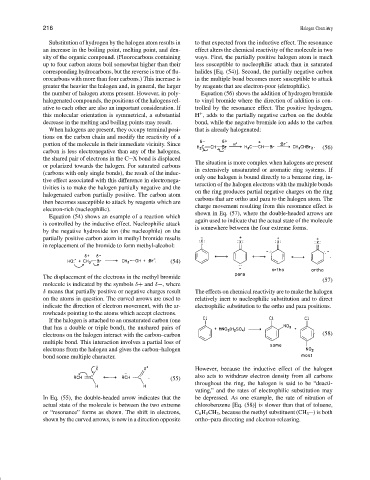
shown in Eq. (57), where the double-headed arrows are
Equation (54) shows an example of a reaction which
again used to indicate that the actual state of the molecule
is controlled by the inductive effect. Nucleophilic attack
is somewhere between the four extreme forms.
by the negative hydroxide ion (the nucleophile) on the
partially positive carbon atom in methyl bromide results
in replacement of the bromide to form methyl-alcohol:
(54)
The displacement of the electrons in the methyl bromide
(57)
molecule is indicated by the symbols δ+ and δ−, where
δ means that partially positive or negative charges result The effects on chemical reactivity are to make the halogen
on the atoms in question. The curved arrows are used to relatively inert to nucleophilic substitution and to direct
indicate the direction of electron movement, with the ar- electrophilic substitution to the ortho and para positions.
rowheads pointing to the atoms which accept electrons.
If the halogen is attached to an unsaturated carbon (one
that has a double or triple bond), the unshared pairs of
electrons on the halogen interact with the carbon–carbon (58)
multiple bond. This interaction involves a partial loss of
electrons from the halogen and gives the carbon–halogen
bond some multiple character.
However, because the inductive effect of the halogen
also acts to withdraw electron density from all carbons
(55)
throughout the ring, the halogen is said to be “deacti-
vating,” and the rates of electrophilic substitution may
In Eq. (55), the double-headed arrow indicates that the be depressed. As one example, the rate of nitration of
actual state of the molecule is between the two extreme chlorobenzene [Eq. (58)] is slower than that of toluene,
or “resonance” forms as shown. The shift in electrons, C 6 H 5 CH 3 , because the methyl substituent (CH 3 ) is both
shown by the curved arrows, is now in a direction opposite ortho–para directing and electron-releasing.