Page 177 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 177
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN007D-343 July 10, 2001 20:13
Inorganic Exotic Molecules 835
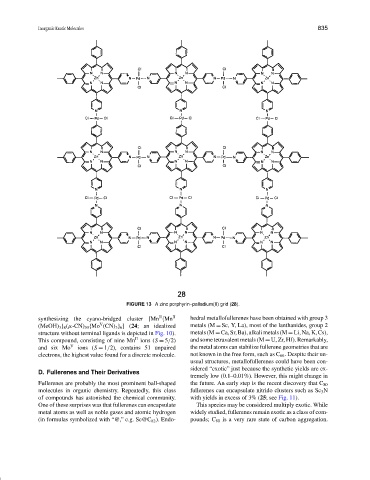
FIGURE 13 A zinc porphyrin–palladium(II) grid (28).
II
synthesizing the cyano-bridged cluster [Mn {Mn II hedral metallofullerenes have been obtained with group 3
V
(MeOH) 3 } 8 (µ-CN) 30 {Mo (CN) 3 } 6 ](24; an idealized metals (M = Sc, Y, La), most of the lanthanides, group 2
structure without terminal ligands is depicted in Fig. 10). metals (M = Ca, Sr, Ba), alkali metals (M = Li, Na, K, Cs),
II
This compound, consisting of nine Mn ions (S = 5/2) and some tetravalent metals (M = U, Zr, Hf). Remarkably,
and six Mo V ions (S = 1/2), contains 51 unpaired the metal atoms can stabilize fullerene geometries that are
electrons, the highest value found for a discrete molecule. not known in the free form, such as C 66 . Despite their un-
usual structures, metallofullerenes could have been con-
sidered “exotic” just because the synthetic yields are ex-
D. Fullerenes and Their Derivatives
tremely low (0.1–0.01%). However, this might change in
Fullerenes are probably the most prominent ball-shaped the future. An early step is the recent discovery that C 80
molecules in organic chemistry. Repeatedly, this class fullerenes can encapsulate nitrido clusters such as Sc 3 N
of compounds has astonished the chemical community. with yields in excess of 3% (25; see Fig. 11).
One of these surprises was that fullerenes can encapsulate This species may be considered multiply exotic. While
metal atoms as well as noble gases and atomic hydrogen widely studied, fullerenes remain exotic as a class of com-
(in formulas symbolized with “@,” e.g. Sc@C 82 ). Endo- pounds; C 80 is a very rare state of carbon aggregation.