Page 118 - Engineering Plastics Handbook
P. 118
92 Engineering Plastics
resistance. Both polyacetals can be used for potable water applications at
82.2°C (180°F), and for repeated food contact at 121°C (250°F). Copolymers,
having long-term heat stability, are used for continuous hot water expo-
sure applications. Introduction of an ethylene link to produce acetal copoly-
mers increases thermal stability and toughness elongation. Unmodified
homopolymers and copolymer, are not resistant to UV and other forms of
SO ) and
radiation, or to concentrated acids such as sulfuric acid (H 2 4
hydrochloric acid (HCl).
Acetal copolymer resins have better long-term thermal stability, but
heat stabilizers can be compounded into homopolymers to increase heat
stability. Acetal copolymers have a UL electrical rating for 100°C (212°F)
service, making the copolymers candidates for long-term electrical appli-
cation service.
Polyacetal resin and compound suppliers recommend that customers
switching between the acetal homopolymer and copolymer consult the
producer, because there can be differences in processing and selective
property considerations. Designers are involved in any polyacetal resin
or compound switch, for property and mold shrinkage differences.
Copolymers have lower centerline porosity in extruded products,
which enhances color uniformity and structural integrity, minimizes
leakage of gases and liquids, and reduces or eliminates areas for bac-
terial growth [16]. With extrusion, porosity is caused by shrinkage
during cooling when the skin cools faster than the core, resulting in
porosity [16].
Chemical resistance (unmodified grades)
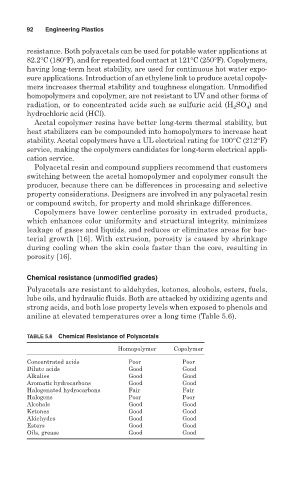
Polyacetals are resistant to aldehydes, ketones, alcohols, esters, fuels,
lube oils, and hydraulic fluids. Both are attacked by oxidizing agents and
strong acids, and both lose property levels when exposed to phenols and
aniline at elevated temperatures over a long time (Table 5.6).
TABLE 5.6 Chemical Resistance of Polyacetals
Homopolymer Copolymer
Concentrated acids Poor Poor
Dilute acids Good Good
Alkalies Good Good
Aromatic hydrocarbons Good Good
Halogenated hydrocarbons Fair Fair
Halogens Poor Poor
Alcohols Good Good
Ketones Good Good
Aldehydes Good Good
Esters Good Good
Oils, grease Good Good