Page 469 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 469
Air injection 433
13.4.2.1 Pressure effect
It has been observed that as the system pressure is increased, the reaction rate
is increased, more heat is released, and the reactions move to lower temper-
atures (Bae, 1977; Yoshiki and Phillips, 1985; Nickle et al., 1987; Li et al.,
2006). It is believed that higher pressure extends to the flammable limits of
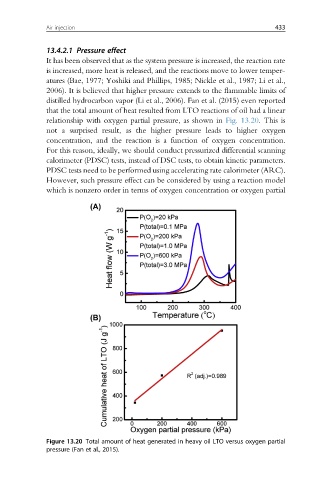
distilled hydrocarbon vapor (Li et al., 2006). Fan et al. (2015) even reported
that the total amount of heat resulted from LTO reactions of oil had a linear
relationship with oxygen partial pressure, as shown in Fig. 13.20. This is
not a surprised result, as the higher pressure leads to higher oxygen
concentration, and the reaction is a function of oxygen concentration.
For this reason, ideally, we should conduct pressurized differential scanning
calorimeter (PDSC) tests, instead of DSC tests, to obtain kinetic parameters.
PDSC tests need to be performed using accelerating rate calorimeter (ARC).
However, such pressure effect can be considered by using a reaction model
which is nonzero order in terms of oxygen concentration or oxygen partial
Figure 13.20 Total amount of heat generated in heavy oil LTO versus oxygen partial
pressure (Fan et al., 2015).