Page 197 - Environmental Control in Petroleum Engineering
P. 197
Waste Treatment Methods 183
Precipitation
Many dissolved solids precipitate from water to form scale as the
temperature, pressure, and/or chemistry changes. The most widely used
system for precipitation is to add lime (CaOH) or sodium hydroxide
(NaOH) to increase the pH of the water. At high pHs, dissolved solids,
including heavy metals, tend to precipitate as a hydroxide sludge. Lime
plus sodium carbonate can also be used to enhance the precipitation
of calcium carbonate. The pH at which many metal hydroxides will
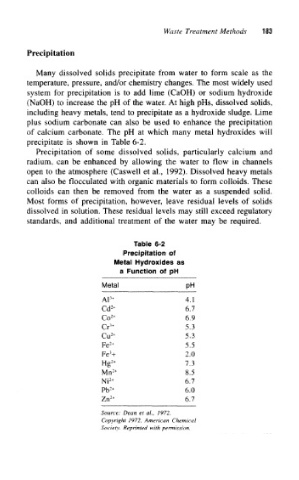
precipitate is shown in Table 6-2.
Precipitation of some dissolved solids, particularly calcium and
radium, can be enhanced by allowing the water to flow in channels
open to the atmosphere (Caswell et al., 1992). Dissolved heavy metals
can also be flocculated with organic materials to form colloids. These
colloids can then be removed from the water as a suspended solid.
Most forms of precipitation, however, leave residual levels of solids
dissolved in solution. These residual levels may still exceed regulatory
standards, and additional treatment of the water may be required.
Table 6-2
Precipitation of
Metal Hydroxides as
a Function of pH
Metal pH
AP 4.1
Cd 2+ 6.7
Co 2+ 6.9
Cr 3+ 5.3
Cu 2+ 5.3
Fe 2+ 5.5
3
Fe + 2.0
Hg 2+ 7.3
Mn 2+ 8.5
2+
Ni 6.7
2+
Pb 6.0
Zn 2+ 6.7
Source: Dean et al., 1972,
Copyright 1972, American Chemical
Society. Reprinted with permission.