Page 361 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 361
346 Environmental Applications of Nanomaterials
Application to dense membranes:
Reverse osmosis
Reverse osmosis membranes are permeable to water while rejecting, to
a large extent, solutes present in the feed. If, in the absence of any
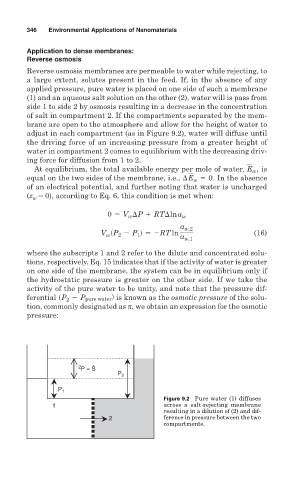
applied pressure, pure water is placed on one side of such a membrane
(1) and an aqueous salt solution on the other (2), water will is pass from
side 1 to side 2 by osmosis resulting in a decrease in the concentration
of salt in compartment 2. If the compartments separated by the mem-
brane are open to the atmosphere and allow for the height of water to
adjust in each compartment (as in Figure 9.2), water will diffuse until
the driving force of an increasing pressure from a greater height of
water in compartment 2 comes to equilibrium with the decreasing driv-
ing force for diffusion from 1 to 2.
At equilibrium, the total available energy per mole of water, E w , is
equal on the two sides of the membrane, i.e., E 5 0 . In the absence
w
of an electrical potential, and further noting that water is uncharged
(z w 0), according to Eq. 6, this condition is met when:
0 5 V P 1 RT ln a w
w
a w,2
V sP 2 P d 52RT ln a w,1 (16)
w
2
1
where the subscripts 1 and 2 refer to the dilute and concentrated solu-
tions, respectively. Eq. 15 indicates that if the activity of water is greater
on one side of the membrane, the system can be in equilibrium only if
the hydrostatic pressure is greater on the other side. If we take the
activity of the pure water to be unity, and note that the pressure dif-
d
ferential sP 2 Ppure water is known as the osmotic pressure of the solu-
2
tion, commonly designated as , we obtain an expression for the osmotic
pressure:
2
P = S
P 2
P 1
Figure 9.2 Pure water (1) diffuses
1 across a salt-rejecting membrane
resulting in a dilution of (2) and dif-
2 ference in pressure between the two
compartments.