Page 44 - Essentials of physical chemistry
P. 44
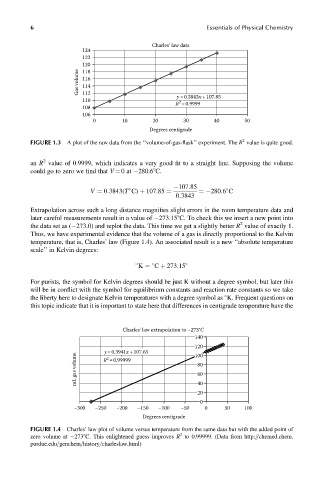
6 Essentials of Physical Chemistry
Charles’ law data
124
122
120
Gas volume 116
118
114
112
y =0.3843x +107.85
110
2
R =0.9999
108
106
0 10 20 30 40 50
Degrees centigrade
2
FIGURE 1.3 A plot of the raw data from the ‘‘volume-of-gas-flask’’ experiment. The R value is quite good.
2
an R value of 0.9999, which indicates a very good fit to a straight line. Supposing the volume
could go to zero we find that V ¼ 0at 280.68C.
107:85
¼ 280:6 C
V ¼ 0:3843(T C) þ 107:85 ¼
0:3843
Extrapolation across such a long distance magnifies slight errors in the room temperature data and
later careful measurements result in a value of 273.158C. To check this we insert a new point into
2
the data set as ( 273.0) and replot the data. This time we get a slightly better R value of exactly 1.
Thus, we have experimental evidence that the volume of a gas is directly proportional to the Kelvin
temperature, that is, Charles’ law (Figure 1.4). An associated result is a new ‘‘absolute temperature
scale’’ in Kelvin degrees:
K ¼ C þ 273:15
For purists, the symbol for Kelvin degrees should be just K without a degree symbol, but later this
will be in conflict with the symbol for equilibrium constants and reaction rate constants so we take
the liberty here to designate Kelvin temperatures with a degree symbol as K. Frequent questions on
this topic indicate that it is important to state here that differences in centigrade temperature have the
Charles’ law extrapolation to –273°C
140
120
mL gas volume R =0.99999 80
y =0.3941x +107.65
100
2
60
40
20
0
–300 –250 –200 –150 –100 –50 0 50 100
Degrees centigrade
FIGURE 1.4 Charles’ law plot of volume versus temperature from the same data but with the added point of
2
zero volume at 2738C. This enlightened guess improves R to 0.99999. (Data from http:==chemed.chem.
purdue.edu=genchem=history=charleslaw.html)