Page 115 - Fluid Catalytic Cracking Handbook
P. 115
92 Fluid Catalytic Cracking Handbook
90
1520°F, 20% steam in air.
80
0 10 20 30 40 50 60 70 80 90 100
Time, hrs
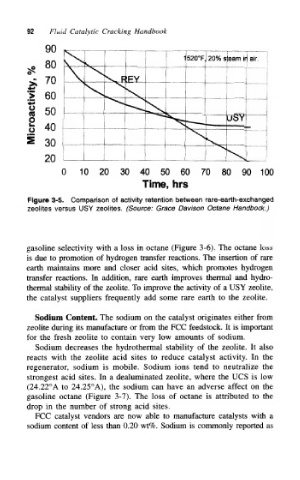
Figure 3-5. Comparison of activity retention between rare-earth-exchanged
zeolites versus USY zeolites. (Source: Grace Davison Octane Handbook.)
gasoline selectivity with a loss in octane (Figure 3-6). The octane loss
is due to promotion of hydrogen transfer reactions. The insertion of rare
earth maintains more and closer acid sites, which promotes hydrogen
transfer reactions. In addition, rare earth improves thermal and hydro-
thermal stability of the zeolite. To improve the activity of a USY zeolite,
the catalyst suppliers frequently add some rare earth to the zeolite.
Sodium Content. The sodium on the catalyst originates either from
zeolite during its manufacture or from the FCC feedstock. It is important
for the fresh zeolite to contain very low amounts of sodium.
Sodium decreases the hydrothermal stability of the zeolite. It also
reacts with the zeolite acid sites to reduce catalyst activity. In the
regenerator, sodium is mobile. Sodium ions tend to neutralize the
strongest acid sites. In a dealuminated zeolite, where the UCS is low
(24.22°A to 24.25°A), the sodium can have an adverse affect on the
gasoline octane (Figure 3-7). The loss of octane is attributed to the
drop in the number of strong acid sites.
FCC catalyst vendors are now able to manufacture catalysts with a
sodium content of less than 0.20 wt%. Sodium is commonly reported as