Page 26 - Instant notes
P. 26
Physical Chemistry 12
to interact close to the point of collision, and so comply with this assumption. Because
the intermolecular interactions become important for real gases at moderate and high
pressures, they are non-ideal gases and they no longer conform to the ideal gas laws.
At intermediate pressures, attractive forces dominate the molecular interactions, and
the volume of the gas becomes lower than the ideal gas laws would predict. As
progressively higher pressures are reached, the molecules increase their proximity to one
another and repulsive forces now dominate the intermolecular interactions. At high
pressures, all gases have a higher volume than the ideal gas law predicts, and are much
less compressible.
The compression factor, Z expresses this behavior, and is commonly plotted as a
function of pressure. It is defined as:
where V m is the molar volume.
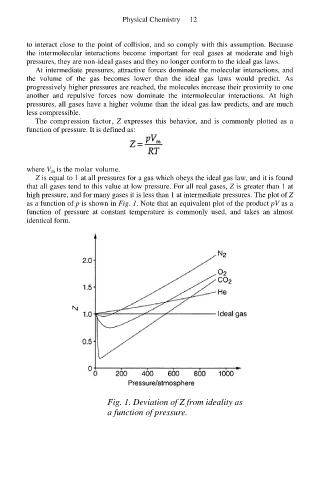
Z is equal to 1 at all pressures for a gas which obeys the ideal gas law, and it is found
that all gases tend to this value at low pressure. For all real gases, Z is greater than 1 at
high pressure, and for many gases it is less than 1 at intermediate pressures. The plot of Z
as a function of p is shown in Fig. 1. Note that an equivalent plot of the product pV as a
function of pressure at constant temperature is commonly used, and takes an almost
identical form.
Fig. 1. Deviation of Z from ideality as
a function of pressure.